This Week in Biotech #47
Catch up on the latest biotech breakthroughs and upcoming trends (Apr 4-10).
Welcome or welcome back to This Week in Biotech by Biotech Blueprint, edition 47.
Make sure to read this week’s deep dive on how tariffs may impact biotech and pharma:
What a global trade fight means for biotech
Scientific research doesn’t respect borders and neither does the biotech supply chain. But trade policy does. With newly implemented tariffs adding cost and friction to the import of lab equipment, reagents, and other R&D essentials, the industry is already adjusting. So far, finished pharmaceutical products have been left off the list. But that exempti…
THIS WEEK’S KEY TAKEAWAYS 🔑
This week in biotech was anything but smooth. Between FDA layoffs, tariff drama, and a handful of big trial updates kept the sector on edge. Markets bounced after Trump hit pause on his sweeping tariff plan, but drugmakers were left wondering what’s next, especially with pharma still on the table. Biotech ETFs like XBI and IBB stayed under pressure as investors tried to navigate an increasingly unpredictable regulatory and trade environment.
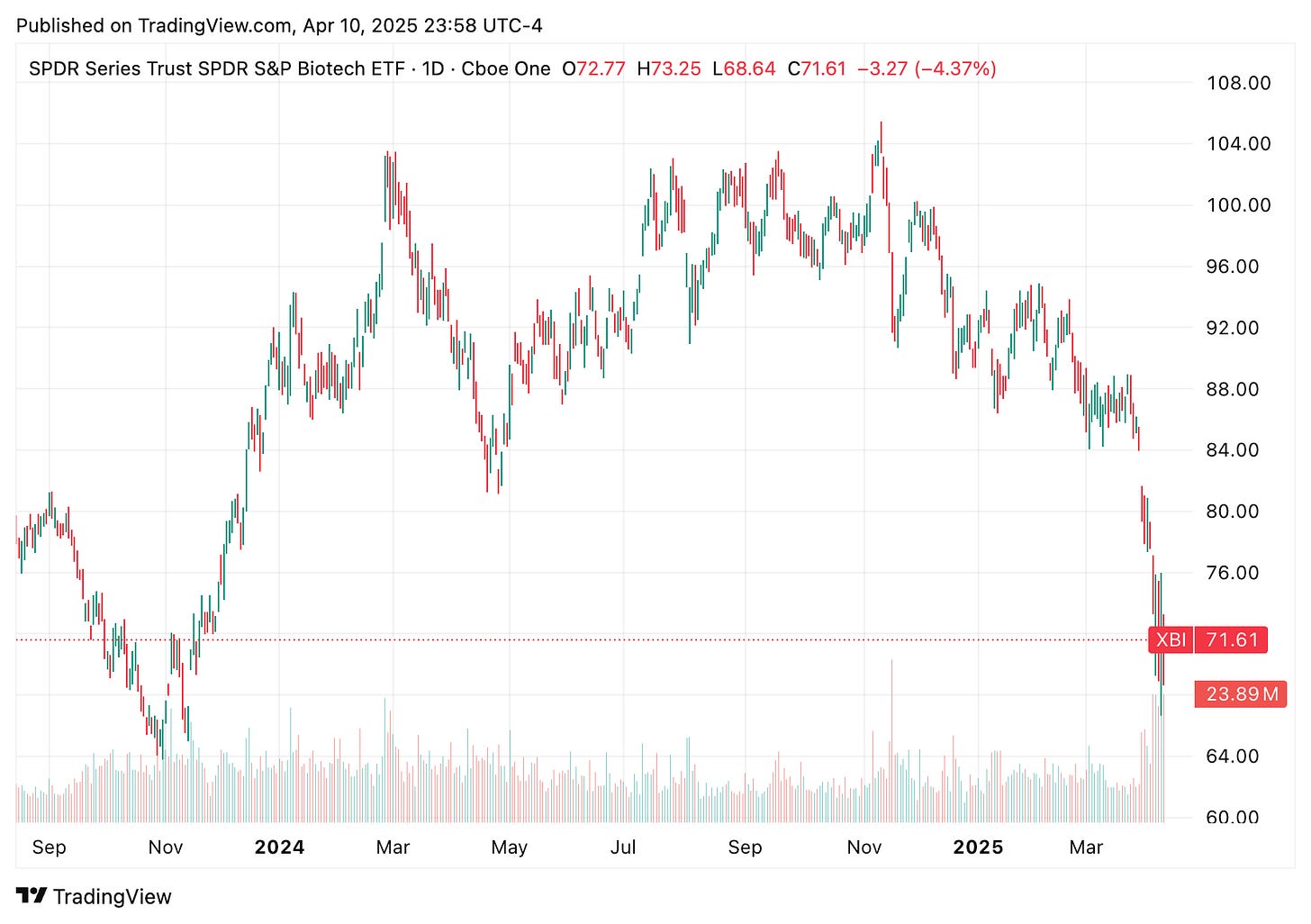
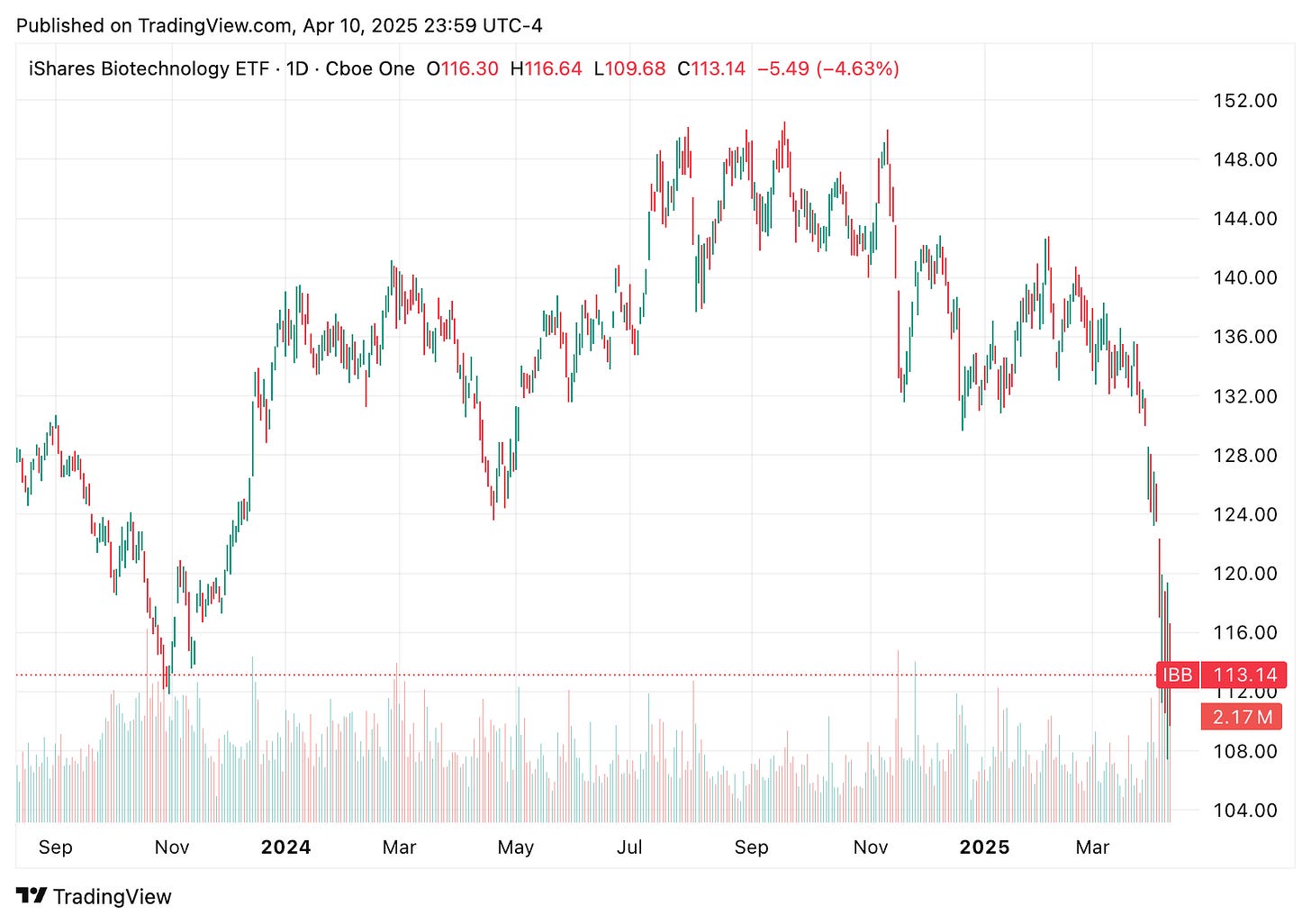
This week brought a major shake up at the Department of Health and Human Services that led to 3,500 job cuts at the FDA. Biotech execs and investors are worried this could seriously slow down drug approvals and cause broader regulatory delays. Health Secretary RFK Jr. also publicly criticized Novavax’s COVID vaccine, calling out its single antigen design as ineffective for respiratory viruses. The comments hit hard, sending NVAX stock tumbling and adding fuel to the debate over where U.S. vaccine strategy is heading next.
Even as the FDA faces staff cuts, Arcturus’ H5N1 mRNA flu vaccine secured fast track status, showing progress hasn’t stalled. At the same time, Trump’s talk of hitting pharmaceutical imports with steep tariffs stirred things up. Even though there’s a 90 day pause in place, the message was clear: bring drug manufacturing back home. That sent European pharma stocks sliding and had companies revisiting their backup plans. Between shifting trade policy and changes at home, the biotech world is navigating a tricky new landscape that could impact where and how companies invest in innovation.
On the clinical side, there were a few hopeful signs. Alzheon’s Alzheimer’s drug didn’t hit its main goal but showed some real promise in high-risk APOE4/4 patients. Sellas reported strong survival gains in AML, which could be a big step forward for its CDK9 inhibitor. And Rhythm Pharma came through with solid phase 3 results in hypothalamic obesity.
Finally, in a surprising pivot, RFK Jr. publicly encouraged measles vaccination amid a growing outbreak in Texas and 21 other states, which a notable departure from his past rhetoric. Time will tell if this marks a sustained shift in tone.
Read more for this week’s updates or listen to the podcast below.
🎙️ PODCAST
MARKET UPDATES
🔹 The biotechnology sector has recently experienced significant turbulence due to a confluence of regulatory changes, trade policies, and market dynamics. The SPDR Biotech ETF (XBI) and the iShares Nasdaq Biotechnology ETF (IBB) have both seen declines, reflecting investor apprehension. Contributing factors include substantial staff reductions at the FDA, leading to concerns about drug approval timelines and regulatory oversight. Additionally, the Trump administration’s imposition of tariffs has raised fears of drug shortages and increased costs.
BIOTECH NEWS
🔹 Shares of Inotiv and Charles River Laboratories plunged 49.5% and 28.1%, respectively, after the FDA announced a major policy shift to reduce reliance on animal testing in drug development. The agency plans to encourage the use of new approach methodologies, including AI models, organoid systems, and real world data from abroad. The move aims to modernize drug evaluation, speed up development, and improve safety while cutting costs. The change has major implications for companies that rely heavily on animal testing services, as investors reevaluate their long term viability.
🔹 Biotech leaders are warning that massive FDA staff cuts are already disrupting regulatory operations and threatening the agency’s effectiveness. In a letter to Senate HELP Committee Chair Bill Cassidy, a group of biotech CEOs, investors, and patient advocates called for urgent oversight following the departure of 3.5k FDA employees, part of a broader HHS restructuring that cut up to 10k roles. The group cited delayed reviews, vacant leadership positions, and the loss of institutional knowledge as major concerns. High-profile resignations, including CDER Director Patrizia Cavazzoni and CBER head Peter Marks, have deepened the unease. The signatories urged Cassidy to assess how the layoffs are affecting the FDA’s core functions and to ensure the agency retains enough expertise to remain a global regulatory leader.
🔹 Novavax shares fell sharply (~24%) on Apr. 10 after U.S. Health Secretary RFK Jr. publicly questioned the efficacy of its COVID-19 vaccine during a CBS News interview. Kennedy attributed the FDA’s delay in granting full approval for the shot, which is currently authorized under emergency use, to its single-antigen design, which he claimed has historically been ineffective for respiratory illnesses. He added that federal health agencies, including the NIH, are now prioritizing development of multi-antigen vaccines instead. The comments come amid broader regulatory uncertainty following the recent resignation of top FDA vaccine official Dr. Peter Marks, further clouding Novavax’s near-term outlook.
🔹 Arcturus Therapeutics has received Fast Track designation from the FDA for its self-amplifying mRNA vaccine candidate, ARCT-2304, aimed at protecting against pandemic influenza A H5N1. The designation is intended to accelerate the development and review of the vaccine, which began phase 1 trials in Nov. 2024. ARCT-2304 uses Arcturus’ proprietary delivery technologies to enhance immune response with lower doses and simpler cold storage requirements. The project is supported by federal funding through BARDA.
🔹 Donald Trump announced that “major” tariffs on pharmaceutical imports are coming, aimed at bringing drug manufacturing back to the U.S., but markets rebounded on Apr. 9 after he issued a 90-day pause on most global tariffs, excluding China.
Speaking at a National Republican Congressional Committee dinner, Trump said drugmakers would soon face steep tariffs, claiming the move would compel companies to relocate manufacturing from countries like China to the U.S. Pharmaceutical imports were initially spared from earlier tariff rounds, but Trump now vows they “will not be spared.” While details and timing remain unclear, the announcement initially spooked markets and sent shares of major European pharma companies like AstraZeneca, GSK, Roche, and Novartis sharply lower. However, just hours after global tariffs went into effect, Trump reversed course announcing a 90-day pause for most countries, though tariffs on Chinese goods were raised to 125% following Beijing’s retaliatory actions.
The shift sent U.S. markets soaring, with the S&P 500 posting its best single-day performance since 2008. Pharma stocks also rebounded as fears of immediate pricing pressure eased. While the pharmaceutical sector has a temporary reprieve, uncertainty around tariff policy continues to hang over global drugmakers.
🔹 AbbVie has announced that the European Commission has approved Rinvoq (upadacitinib) for the treatment of adults with giant cell arteritis (GCA), making it the first and only oral JAK inhibitor authorized for this condition in the EU. The approval is based on results from the phase 3 trial, which showed Rinvoq helped more patients achieve sustained remission and reduced disease flares and steroid use compared to placebo. GCA is a serious autoimmune condition that affects large and medium arteries, mostly in people 50+. This marks the eighth approved indication for Rinvoq in the EU.
🔹 The European Commission has approved a new under-the-skin (subcutaneous) version of Rybrevant (amivantamab), a targeted cancer therapy for patients with advanced non-small cell lung cancer (NSCLC) driven by EGFR mutations. Co-developed by J&J and Halozyme using the Enhanze drug delivery platform, the new formulation can be given more quickly and with fewer infusion-related side effects. It’s approved for use with lazertinib as a first-line treatment for common EGFR mutations, and on its own for patients with exon 20 insertions who have already received chemotherapy. This marks the tenth regulatory approval for a therapy using Halozyme’s Enhanze technology.
🔹 European pharmaceutical companies are reassessing strategies to mitigate the potential impact of new U.S. tariffs, as President Trump signaled plans to include pharmaceuticals in future trade actions to encourage domestic drug manufacturing. Although pharma has been exempt from tariffs under a 1994 WTO agreement, that protection may soon end, putting pressure on firms with major operations in Ireland, Germany, and Switzerland. Companies like Novartis, Merck, and Roche are evaluating U.S. policy changes and developing contingency plans, while others such as Sanofi, GSK, and AstraZeneca are likely to face challenges. Analysts warn that tariffs could reduce profit margins and stifle R&D, but note that many firms already maintain significant U.S. operations. Industry lobby PhRMA expressed support for domestic production goals, while experts suggest AI-driven supply chain optimization and increased U.S. investments may help offset risks.
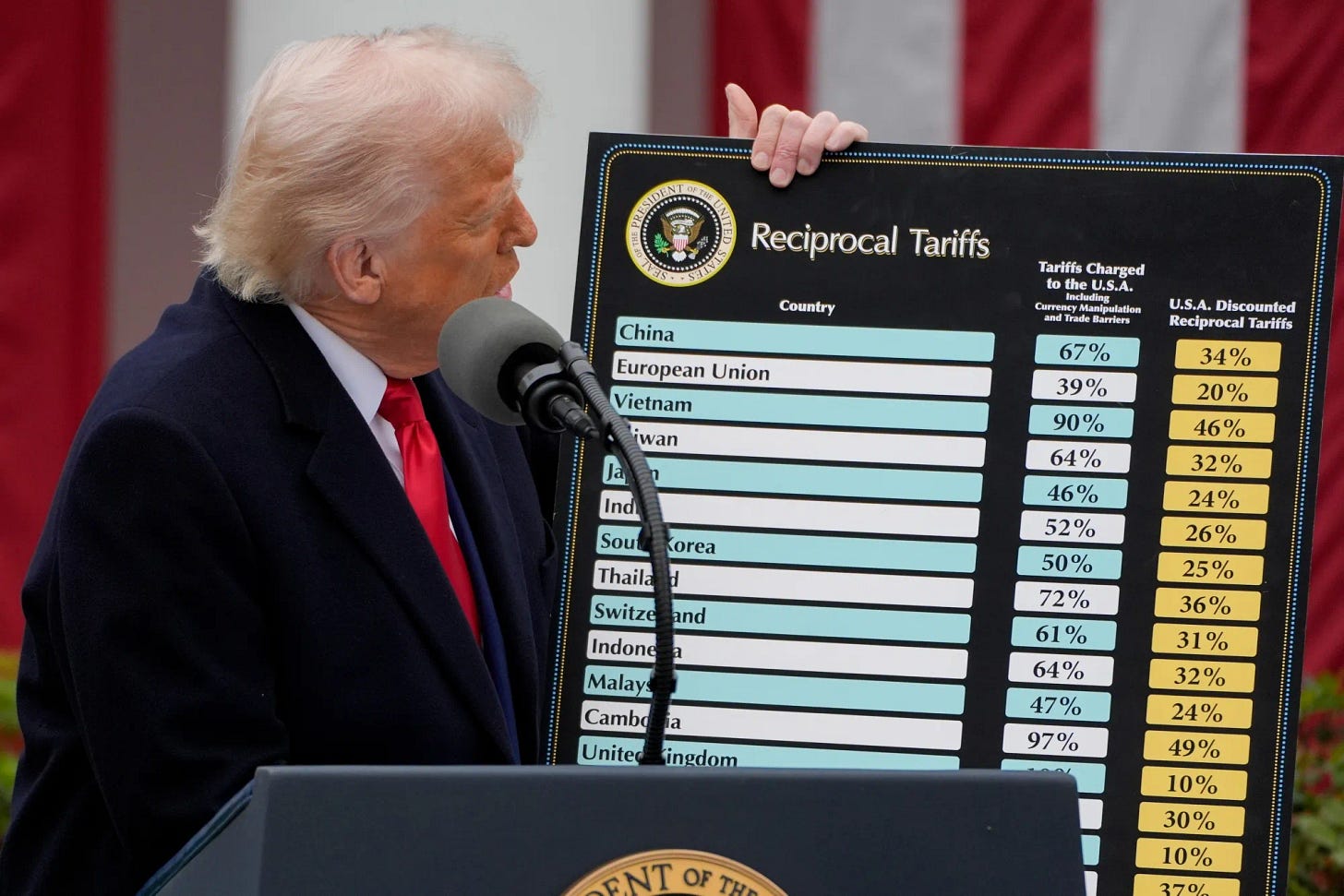
Read my deep dive on how these tariffs could reshape research and biotech innovation in the U.S.👇
CLINICAL TRIAL UPDATES
🔹 Alzheon’s phase 3 APOLLOE4 trial of valiltramiprosate (ALZ-801) in early Alzheimer’s patients with two APOE4 gene copies did not meet its primary endpoint in the overall population. However, in patients at the mild cognitive impairment stage, the drug showed nominally significant cognitive and functional benefits, along with slowed brain atrophy. The treatment was well-tolerated with no serious safety issues. Alzheon plans to continue development, focusing on this high-risk APOE4/4 subgroup.
🔹 SELLAS Life Sciences announced promising results from its ongoing phase 2 trial of SLS009 in relapsed or refractory acute myeloid leukemia (AML). In the study, patients with AML with myelodysplasia-related changes (AML-MRC) achieved a median overall survival of 8.9 months, while those previously treated with venetoclax-based regimens saw a median survival of 8.8 months, which was well above the historical benchmark of 2.5 months. The overall response rate in AML-MRC patients reached 67%, more than triple the company’s original goal of 20%. SLS009 has continued to demonstrate a favorable safety profile, with no new safety concerns observed. SELLAS expects to receive regulatory feedback from the FDA in the first half of 2025.
🔹 Rhythm Pharmaceuticals’ stock surged over 17% following the announcement that its pivotal phase 3 trial met its primary endpoint in treating acquired hypothalamic obesity with setmelanotide. The study demonstrated a statistically significant placebo-adjusted BMI reduction of -19.8% at 52 weeks across 120 patients, with consistent efficacy in both adult and pediatric populations. Over 80% of patients receiving setmelanotide achieved at least a 5% BMI reduction, and no new safety signals emerged. These positive results pave the way for regulatory submissions in the U.S. and EU later this year.
PUBLIC HEALTH SPOTLIGHT
🔹 In his first network TV interview since becoming Health and Human Services Secretary, Robert F. Kennedy Jr. encouraged people to get the measles vaccine, marking a notable shift in tone amid a growing outbreak in West Texas that has killed two children and infected over 500 people. Speaking with CBS News’ Dr. Jon LaPook, Kennedy affirmed that both his and the federal government’s position is that people should get vaccinated, though he maintained his stance against vaccine mandates. While he previously acknowledged the MMR vaccine’s effectiveness in writing and on social media, this interview was the first time he directly mentioned vaccination. The current outbreak is the worst in the U.S. since 2019, with health officials stressing that the majority of those infected are unvaccinated. Kennedy, who has faced criticism for past misinformation about vaccines, reiterated that he is not anti-vaccine but advocates for informed consent based on solid science.
ON THE HORIZON
🔹 April 2025 FDA PDUFAs:
Apr. 2: Reproxalap is an investigational drug developed by Aldeyra Therapeutics for the treatment of dry eye disease. It functions as a small-molecule modulator of reactive aldehyde species, which are elevated in ocular and systemic inflammatory diseases. REJECTED ❌
Apr. 3: Cabozantinib, marketed by Exelixis under the brand name Cabometyx, is an oral tyrosine kinase inhibitor approved for treating various cancers, including advanced renal cell carcinoma, hepatocellular carcinoma, and differentiated thyroid cancer.
Apr. 3: Amgen's (Horizon Therapeutics) Inebilizumab (Uplizna) for IgG4-related disease (supplemental BLA for the CD19-targeted mAb’s new use). APPROVED ✅
Apr. 18: Dupilumab (Dupixent), developed by Regeneron and Sanofi, is an anti-inflammatory biologic already approved for several conditions like atopic dermatitis, asthma, and eosinophilic esophagitis. The FDA is currently reviewing two supplemental applications: one for bullous pemphigoid (PDUFA date: June 20, 2025) and another for chronic spontaneous urticaria (PDUFA date: April 18, 2025), which could further expand its use.
Apr. 26: Telix Pharma’s TLX101-CDx (Pixclara), imaging agent for recurrent glioma (new NDA for F-18 FET diagnostic tracer) awaiting its decision on Apr. 26.
Apr. 29: Elamipretide is an investigational peptide developed by Stealth BioTherapeutics, designed to target mitochondrial dysfunction by binding to cardiolipin in the inner mitochondrial membrane, thereby enhancing mitochondrial function. It is primarily being developed for the treatment of Barth syndrome, an ultra-rare genetic disorder characterized by cardiac abnormalities, muscle weakness, and reduced life expectancy.
Apr. 29: J&J’s Nipocalimab for generalized Myasthenia Gravis (gMG) in adults who are anti-AChR, anti-MuSK or anti-LRP4 antibody-positive (original BLA, anti-FcRn monoclonal antibody). The product has orphan designation and priority review.
Apr. 30: Dihydroergotamine (DHE) is used for the acute treatment of migraine with or without aura in adults. Satsuma Pharmaceuticals is developing STS101, a novel DHE nasal powder formulation designed for fast, easy self-administration and rapid absorption.
Have a great rest of your week and thanks for reading Biotech Blueprint.
👩🏻💻 BIOTECH BLUEPRINT CONSULTING
I have a new website: biotechblueprint.com!
I provide tailored consulting solutions designed to meet the unique challenges of both established companies and startups. My services span a wide range of strategic and technical needs, including:
Research strategy & grant writing
Scientific communication & medical affairs
Data analysis & interpretation
Biotech/pharma innovation & technology assessment
Startup advisory services
I also provide daily Biotech Blueprint newsletters and custom daily analysis (charts & graphs) for individuals and companies.
BOOK A FREE 30-MINUTE CONSULTATION OR A MEET & GREET below.
DISCLAIMER: This content is for informational purposes only. It should not be taken as legal, tax, investment, financial, or other advice. The views expressed here are my own and do not reflect the opinions of any company or institution.
DISCLOSURE: I have no business relationships with any company mentioned in this article.







