This Week in Biotech #46
Catch up on the latest biotech breakthroughs and upcoming trends (Mar 28-Apr 3).
Welcome or welcome back to This Week in Biotech by Biotech Blueprint, edition 46.
THIS WEEK’S KEY TAKEAWAYS 🔑
One of the major news this week was the abrupt resignation of Dr. Peter Marks, the FDA’s top vaccine regulator. And this should set off alarm bells, not just for biotech investors, but for anyone who values a stable, science-based regulatory environment.
His departure, supposedly driven by frustration over misinformation from senior health officials and what he described as the “disregard for scientific truth,” comes amid mass layoffs and reorientation of U.S. health policy under HHS Secretary Robert F. Kennedy Jr.
The consequences have been swift. Moderna shares dropped 9% in one day. BioNTech, Novavax, and others followed. Vaxcyte plunged over 50% despite releasing positive clinical data that same morning.
Investors aren’t spooked because of poor science. They’re spooked because of shifting ground rules. If agencies begin altering approval standards or adding new obstacles without transparency, the message is clear: your billion $ pipeline can evaporate overnight. RFK Jr. has long been critical of vaccines. Now, as the top public health official in the country, his views are no longer theoretical, they might become institutionalized.
Adding to the uncertainty is the growing threat of tariffs on pharmaceuticals. While Trump’s recent trade announcement exempted the sector (for now), White House documents make it clear that medicines could be next. As John Crowley, CEO of BIO, warned in an interview with Endpoints News, these tariffs are an “existential threat.” Ireland, a cornerstone of global pharmaceutical production, stands to be among the most affected. For companies that have spent years and billions building out their operations in Europe, this could be very disruptive.
The push to bring more manufacturing back to the U.S. is understandable and strengthening the domestic supply chain makes sense in principle. But moving drug production isn’t as simple as flipping a switch. It’s a long, costly process, tied up in years of tech transfer and regulatory hurdles. If tariffs are introduced before new sites are ready, or worse, if they’re used as leverage in global trade disputes, we won’t get resilience. We’ll get disruption.
For years, the biotech sector has depended on a careful balance of risk-taking, innovation, and steady collaboration with regulators. That balance is beginning to slip. Just as the industry gains momentum in areas like Alzheimer’s, obesity, cancer, and rare diseases, new challenges are emerging. If FDA leadership remains in flux, if tariffs disrupt long-standing manufacturing networks, and if politics begins to override scientific review, progress may slow.
As John Crowley put it, the industry is “dangerously close” to breaking. The warning shouldn’t be taken lightly. Investors, scientists, and patients would be wise to watch not just market performance, but the shifting ground beneath it. The risk is not just about financial returns, but about the conditions that enable scientific progress to begin with.
If biotech stumbles, it won’t be due to a lack of innovation. It will be because the policy environment stopped working together with the science.
🎙️ PODCAST
MARKET UPDATES
🔹 Vaxcyte (PCVX) shares plunged over 55% on Mar. 31, despite reporting positive mid-stage results for its 24 strain pneumococcal vaccine candidate, VAX-24, in healthy infants. The vaccine met all target immune response criteria for the four additional strains it covers beyond Pfizer’s Prevnar 20 and showed a comparable safety profile. However, investor optimism was overshadowed by broader market concerns following the abrupt resignation of Dr. Peter Marks, the FDA’s top vaccine regulator, who cited “misinformation and lies” from HHS Secretary RFK Jr. as his reason for stepping down. The move, combined with plans for mass layoffs at the FDA, spooked biotech investors, dragging down vaccine-related stocks.
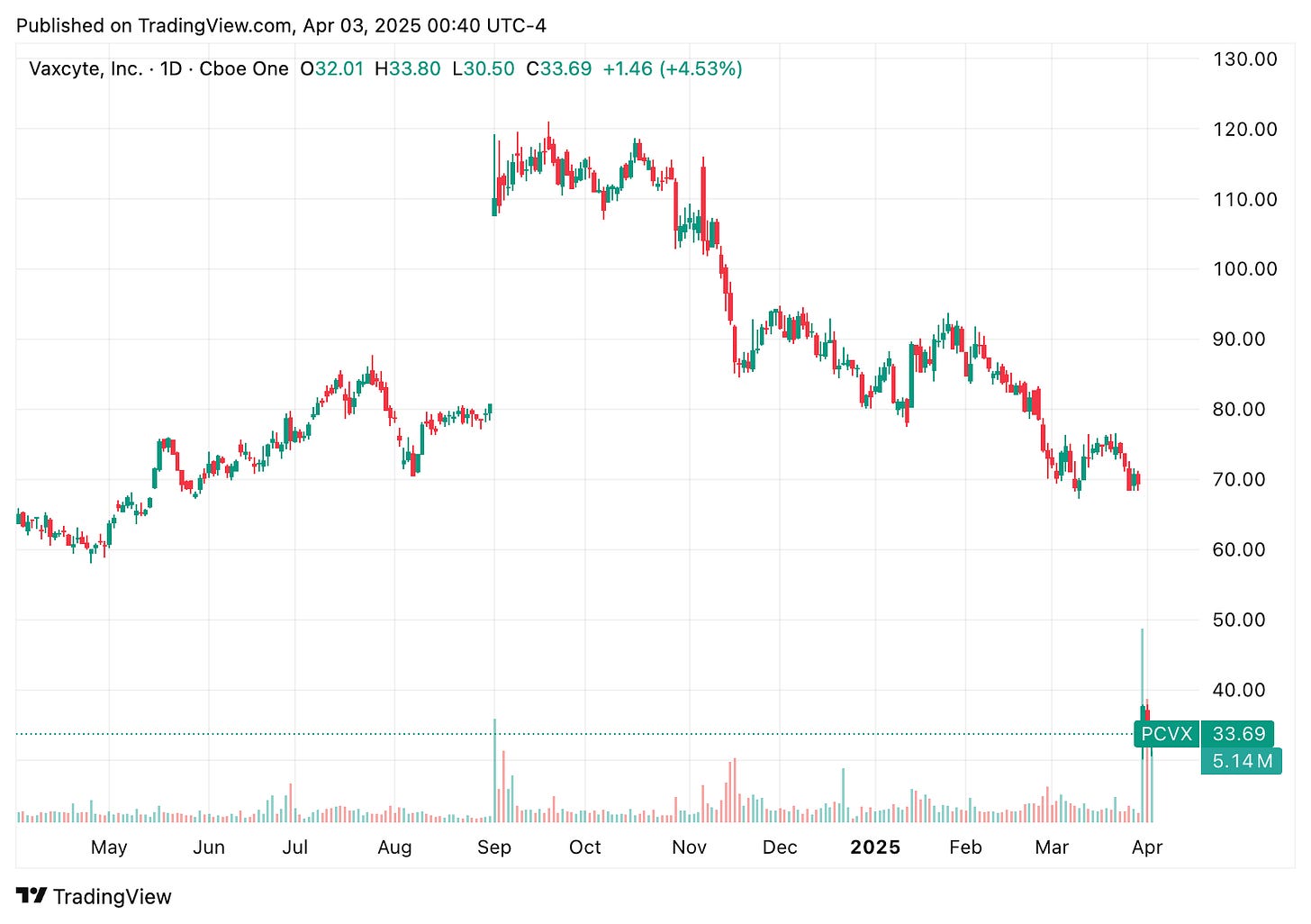
🔹 Vaccine-focused biopharma stocks were under heavy pressure on Monday due to Marks’s resignation, with several major players seeing sharp declines. Moderna (MRNA) was down 9%, BioNTech (BNTX) ~6%, and Novavax (NVAX) and CureVac (CVAC) have dropped 7% and 8%, respectively. Even Vaxcyte (PCVX), despite releasing positive mid-stage data for its pneumococcal vaccine candidate earlier in the day, has plunged >50%.
🔹 Lexicon Pharmaceuticals (LXRX) shares surged 30% over the past week following a licensing deal with Novo Nordisk for its preclinical obesity drug, LX9851. Under the agreement, Lexicon will receive a $75M upfront payment, with potential milestone payments bringing the total deal value up to $1B, along with royalties. This partnership follows Lexicon’s recent pivot to focus on clinical development after discontinuing efforts to seek approval for its type 1 diabetes drug and laying off 60% of its workforce. LX9851, a novel small-molecule targeting fat metabolism, has shown promising preclinical results, especially when combined with Novo’s semaglutide.
BIOTECH NEWS
🔹 Sangamo Therapeutics has signed a licensing agreement with Eli Lilly to use its novel STAC-BBB capsid, which enables effective delivery of genomic medicines across the blood brain barrier. Lilly will initially use the capsid for one central nervous system (CNS) disease target, with rights to expand to four more. Sangamo will receive an $18M upfront payment, and could earn up to $1.4B in milestones and royalties. This is Sangamo’s third pharma partnership since announcing STAC-BBB in 2024, signaling strong industry interest in its CNS gene therapy delivery platform.
🔹 Aldeyra Therapeutics’ New Drug Application for reproxalap was rejected by the FDA yesterday. Reproxalap is an investigational treatment for dry eye disease. The FDA cited insufficient evidence of efficacy and requested an additional well-controlled trial to demonstrate effectiveness in treating ocular symptoms. Although no safety or manufacturing issues were noted, the rejection marks the second setback for reproxalap’s approval. Aldeyra’s stock has plummeted over 73% since the announcement.
🔹 The FDA has missed its decision deadline for Novavax’s COVID-19 vaccine approval, raising concerns across the vaccine and biotech sectors. The vaccine remains available under emergency use authorization, but the delay, linked to mass layoffs at federal health agencies and oversight shifts under HHS Secretary Robert F. Kennedy Jr., is fueling fears of political interference and regulatory uncertainty. Bank of America analysts warned this could signal broader risks for the industry. Novavax shares have fallen over 20% in the past week.
🔹 Valneva’s chikungunya vaccine, Ixchiq, has received European Commission approval for use in adolescents aged 12+, expanding its prior adult indication. Ixchiq is the world’s first approved vaccine for chikungunya and is now authorized in the EU, Norway, Liechtenstein, and Iceland for individuals 12+. This milestone follows positive phase 3 data showing strong and sustained immune response in adolescents.
🔹 At the AD/PD 2025 conference, Roche presented promising advancements in its Alzheimer’s and Parkinson’s disease programs. In Alzheimer’s, the company highlighted new data showing that trontinemab, an investigational bispecific antibody, achieved rapid and deep dose-dependent reductions in amyloid plaques with a favorable safety profile. Based on these results, Roche plans to initiate a phase 3 program for trontinemab later this year. Additionally, Roche’s Elecsys pTau181 plasma test demonstrated strong potential to accurately rule out amyloid pathology using a minimally invasive blood sample, which could reduce the need for more invasive diagnostic tools. A European launch is anticipated by late 2025. In Parkinson’s disease, Roche shared results from the phase 2b trial of prasinezumab, which missed its primary endpoint. Roche is further evaluating the data to determine future development plans.
🔹 Biogen announced that its investigational therapy BIIB080, which targets tau protein in Alzheimer’s disease, has received fast track designation from the FDA. The antisense oligonucleotide therapy showed promising phase 1b results, including reduced tau levels and early signs of clinical benefit. BIIB080 is currently in a fully enrolled phase 2 trial, with data expected in 2026.
🔹 On Apr. 1, the Trump administration began mass layoffs across federal health agencies, cutting around 20k jobs, including at the FDA, NIH, and CDC. Entire teams, including the FDA’s communications staff, were eliminated. The cuts also hit agencies supporting seniors, the disabled, and low-income households. HHS Secretary RFK Jr. defended the move as reducing “bureaucratic sprawl,” but experts warn it threatens public health infrastructure, drug oversight, and critical services for millions.
🔹 Dr. Peter Marks, the FDA's top vaccine official, has resigned effective Apr. 5, citing concerns over misinformation and political interference under Health Secretary Robert F. Kennedy Jr. In his resignation letter, Marks accused Kennedy of promoting “misinformation and lies” about vaccine safety and disregarding scientific truth. Marks, a key figure in the COVID-19 vaccine rollout and creator of Operation Warp Speed, said efforts to address RFK Jr.’s vaccine concerns were futile. His resignation comes amid broader turmoil at HHS, including mass layoffs and agency closures.
🔹 The FDA has approved Sanofi’s Qfitlia (fitusiran) as the first antithrombin-lowering therapy in the U.S. for routine prophylactic treatment of hemophilia A or B, with or without inhibitors, in patients aged 12+. Administered as few as six times a year via subcutaneous injection, Qfitlia significantly reduced annual bleeding rates in clinical trials. It works by lowering antithrombin levels to enhance clotting and is the first hemophilia treatment to use small-interfering RNA technology. While highly effective, it carries risks of adverse events like thrombosis and liver issues. Sanofi will support patients through its HemAssist program and companion diagnostic testing.
🔹 Milestone Pharmaceuticals hit a roadblock after the FDA issued a Complete Response Letter for its lead drug candidate, etripamil, a nasal spray for treating paroxysmal supraventricular tachycardia (PSVT). While the FDA didn’t raise concerns about efficacy or safety, the rejection centered on chemistry, manufacturing, and controls (CMC) issues, leaving the door open for approval if Milestone addresses these gaps in a resubmission. Shares of Milestone tumbled over 60% in pre-market trading.
🔹 Eli Lilly’s Alzheimer’s drug Kisunla (donanemab), which is approved in the U.S., was not recommended for approval by the European Medicines Agency (EMA) due to concerns that the benefits do not outweigh risks such as fatal brain swelling. In a trial of over 1,700 participants, three deaths were potentially linked to the treatment. While Lilly defends the drug’s effectiveness in slowing cognitive decline by up to 35%, the EMA’s Committee for Medicinal Products for Human Use (CHMP) cited safety concerns. Lilly hopes the EMA will reverse its decision, as it did with Biogen’s Leqembi, which was initially rejected but later approved.
CLINICAL TRIAL UPDATES
🔹 BioMarin announced that its phase 3 trial of Palynziq in adolescents (ages 12–17) with phenylketonuria met its primary endpoint, significantly lowering blood phenylalanine levels compared to diet alone. Safety data were consistent with prior studies. Palynziq, already approved for adults, could see label expansion later this year as BioMarin plans to submit the results to global regulators.
🔹 Greenwich LifeSciences announced strong progress in its phase 3 trial for GLSI-100, a breast cancer immunotherapy. Positive preliminary immune response and safety data were reported across both patient arms. Enrollment hit a record pace, with 117 active sites across the U.S. and EU and plans to expand to over 150 globally. The company may convert the non-HLA-A*02 arm into a second phase 3 trial, potentially broadening approval paths. Manufacturing and regulatory planning for U.S. and EU markets is also underway.
🔹 AC Immune reported further positive interim results from its phase 2 trial of ACI-7104.056, an active immunotherapy for early Parkinson’s disease. The treatment significantly boosted anti-alpha-synuclein antibodies (over 20 times higher than placebo after four immunizations) and was well tolerated with no serious safety issues reported. Repeated doses enhanced antibody response, supporting the drug’s “boostability.” Based on upcoming biomarker and pharmacodynamic data expected later in 2025, the company may advance to part 2 of the trial with up to 150 patients.
🔹 Compass Therapeutics announced that its investigational cancer drug tovecimig met the primary endpoint in its ongoing phase 2/3 trial for biliary tract cancer (BTC), showing a statistically significant improvement in overall response rate (17.1%) when combined with paclitaxel, compared to paclitaxel alone (5.3%). Despite the positive data, Compass’s stock tumbled nearly 25%, likely due to investor concerns around the relatively modest efficacy improvement and the immaturity of key secondary outcomes like progression-free and overall survival, which won’t be reported until late 2025. The trial remains ongoing, with tovecimig showing a favorable safety profile.
🔹 OKYO Pharma announced that urcosimod, its eye drop treatment for neuropathic corneal pain (NCP), has shown over 2.5 years of stability in single-use ampoules, which is a key FDA requirement. The drug, currently in a phase 2b trial, was also well-tolerated in earlier studies for dry eye disease, showing strong efficacy and comfort. With no FDA-approved treatment for NCP, urcosimod’s stability and promising results support its potential as a first-in-class therapy.
🔹 Opthea announced it will discontinue development of its wet age-related macular degeneration (wet AMD) drug, sozinibercept, after both phase 3 trials (COAST and ShORe) failed to meet their primary endpoints. The ShORe trial showed no significant visual improvement over standard care with ranibizumab. Although the drug was well tolerated, the disappointing results led to an immediate termination of both studies. Opthea now faces uncertainty about its financial future, including potential liabilities under its funding agreement.
🔹 On Mar. 28, Palatin Technologies announced positive phase 2 results for its oral ulcerative colitis drug, PL8177. After 8 weeks, 33% of treated patients achieved clinical remission vs. 0% on placebo, and 78% showed significant clinical response, with no adverse events. The drug targets colon inflammation without systemic absorption and may offer a safer alternative to current therapies. Palatin is advancing licensing talks with major pharma partners and shifting focus to its obesity drug pipeline.
ON THE HORIZON
🔹 March 2025 FDA PDUFAs:
Mar. 18: NT-501, developed by Neurotech Pharmaceuticals, is a treatment designed for Macular Telangiectasia Type 2 (MacTel), a rare neurodegenerative eye disease that causes progressive central vision loss. APPROVED ✅
Mar. 23: Vutrisiran, RNAi therapeutic developed by Alnylam for the treatment of ATTR amyloidosis with cardiomyopathy (ATTR-CM). APPROVED ✅
Mar. 26: Gepotidacin is a novel, oral antibiotic developed by GSK for the treatment of uncomplicated urinary tract infections (UTIs) and gonorrhea. APPROVED ✅
Mar. 27: Milestone’s Etripamil nasal spray is designed to be self-administered as a nasal spray to rapidly treat episodes of paroxysmal supraventricular tachycardia (PSVT), a type of abnormal heart rhythm. REJECTED ❌
Mar. 28: Sanofi’s Fitusiran, a therapy for hemophilia A or B, used to prevent bleeding in patients, including those with inhibitors. APPROVED ✅
🔹 April 2025 FDA PDUFAs:
Apr. 2: Reproxalap is an investigational drug developed by Aldeyra Therapeutics for the treatment of dry eye disease. It functions as a small-molecule modulator of reactive aldehyde species, which are elevated in ocular and systemic inflammatory diseases. REJECTED ❌
Apr. 3: Cabozantinib, marketed by Exelixis under the brand name Cabometyx, is an oral tyrosine kinase inhibitor approved for treating various cancers, including advanced renal cell carcinoma, hepatocellular carcinoma, and differentiated thyroid cancer.
Apr. 3: Amgen's (Horizon Therapeutics) Inebilizumab (Uplizna) for IgG4-related disease (supplemental BLA for the CD19-targeted mAb’s new use). APPROVED ✅
Apr. 18: Dupilumab (Dupixent), developed by Regeneron and Sanofi, is an anti-inflammatory biologic already approved for several conditions like atopic dermatitis, asthma, and eosinophilic esophagitis. The FDA is currently reviewing two supplemental applications: one for bullous pemphigoid (PDUFA date: June 20, 2025) and another for chronic spontaneous urticaria (PDUFA date: April 18, 2025), which could further expand its use.
Apr. 26: Telix Pharma’s TLX101-CDx (Pixclara), imaging agent for recurrent glioma (new NDA for F-18 FET diagnostic tracer) awaiting its decision on Apr. 26.
Apr. 29: Elamipretide is an investigational peptide developed by Stealth BioTherapeutics, designed to target mitochondrial dysfunction by binding to cardiolipin in the inner mitochondrial membrane, thereby enhancing mitochondrial function. It is primarily being developed for the treatment of Barth syndrome, an ultra-rare genetic disorder characterized by cardiac abnormalities, muscle weakness, and reduced life expectancy.
Apr. 29: J&J’s Nipocalimab for generalized Myasthenia Gravis (gMG) in adults who are anti-AChR, anti-MuSK or anti-LRP4 antibody-positive (original BLA, anti-FcRn monoclonal antibody). The product has orphan designation and priority review.
Apr. 30: Dihydroergotamine (DHE) is used for the acute treatment of migraine with or without aura in adults. Satsuma Pharmaceuticals is developing STS101, a novel DHE nasal powder formulation designed for fast, easy self-administration and rapid absorption.
Have a great rest of your week and thanks for reading Biotech Blueprint.
👩🏻💻 BIOTECH BLUEPRINT CONSULTING
I provide tailored consulting solutions designed to meet the unique challenges of both established companies and startups. My services span a wide range of strategic and technical needs, including:
Research strategy & grant writing
Scientific communication & medical affairs
Data analysis & interpretation
Biotech/pharma innovation & technology assessment
Startup advisory services
I also provide daily Biotech Blueprint newsletters and custom daily analysis (charts & graphs) for individuals and companies.
BOOK A FREE 30-MINUTE CONSULTATION OR A MEET & GREET below.
DISCLAIMER: This content is for informational purposes only. It should not be taken as legal, tax, investment, financial, or other advice. The views expressed here are my own and do not reflect the opinions of any company or institution.
DISCLOSURE: I have no business relationships with any company mentioned in this article.






Nice update