This Week in Biotech #44
Catch up on the latest biotech breakthroughs and upcoming trends (Mar 14-20).
Welcome or welcome back to This Week in Biotech by Biotech Blueprint, edition 44.
THIS WEEK’S KEY TAKEAWAYS 🔑
📉 Gilead shares dipped after reports that HHS may cut HIV prevention funding. Analysts downplayed the impact, noting 70–80% of PrEP sales are commercial. Meanwhile, the FDA is set to decide in June on lenacapavir, Gilead’s long-acting, twice-yearly PrEP shot—seen as a major growth catalyst.
⚠️ A patient death from acute liver failure after treatment with Sarepta’s Elevidys gene therapy sent shares tumbling. While Sarepta maintained this wasn’t a new safety signal, the news sparked concern and volatility across the DMD gene therapy space.
🚀 Regenxbio shares rose after releasing positive interim data from its RGX-202 DMD gene therapy trial, showing strong microdystrophin expression and no serious adverse events, including in children under 4. A BLA filing is targeted for mid-2026.
🧬 Monte Rosa’s VAV1 degrader (MRT-6160) showed >90% target knockdown and strong immune modulation in a phase 1 trial, with Novartis backing phase 2.
✅ The FDA approved Alnylam’s Amvuttra (vutrisiran) for ATTR amyloidosis with cardiomyopathy, expanding its label. The RNAi therapy is now the first to treat both polyneuropathy and cardiac manifestations of the disease, and is administered via quarterly injections.
📉 After disappointing phase 1 results for EO-3021, Elevation is scrapping the program and shifting its attention to HER3-targeting ADC EO-1022. An IND is expected in 2026. The company is also exploring strategic options following a >40% drop in share price.
💉 While batoclimab met endpoints in both MG and CIDP, Immunovant will not pursue approval. Instead, it will advance IMVT-1402, a next-generation FcRn inhibitor, which could offer deeper and more durable responses across autoimmune diseases. 💉
🧠 Scholar Rock’s Apitegromab significantly improved motor function in SMA patients already on SMN therapy. No serious adverse events were reported, underscoring its potential as a muscle-targeted, first-in-class treatment.
🎙️ PODCAST
MARKET UPDATES
🔹 Gilead Sciences’ stock fell 2.5% after reports surfaced that the Department of HHS may cut federal HIV prevention funding, a key area for Gilead, which generated $19.6B in HIV-related sales last year. While the news raised concerns, analysts downplayed the impact, noting that 70–80% of Gilead’s PrEP sales come from the commercial market, not federally funded programs. The CDC’s role is focused on research and education rather than drug reimbursement. Gilead is also preparing to launch lenacapavir, a twice-yearly injectable PrEP drug, with an FDA decision expected in June, which analysts see as a major future growth driver.
🔹 Moderna remained the most shorted healthcare stock in February, with short interest rising to 13.19% from 11.84% in January. The company received a Strong sell rating from Seeking Alpha’s quant ratings with a score of 1.17 out of 5, following a 65% year-over-year revenue decline in Q4 2024 and a weaker than expected 2025 sales forecast. However, as a scientist, I take a bottom-up approach, focusing on the potential of drug candidates rather than overall market sentiment. Moderna’s mRNA platform is at the forefront of a medical revolution, with potential applications in oncology, rare diseases, and personalized medicine. The company is targeting up to 10 product approvals by 2027, and I believe its pipeline has the potential to drive massive breakthroughs. While short term financial challenges persist, I see Moderna as a leader in the next era of mRNA therapeutics.
🔹 Sarepta Therapeutics’ stock fell up to 25% after the company reported the death of a Duchenne muscular dystrophy patient from acute liver failure following treatment with its gene therapy, Elevidys. This marks the first such fatality among over 800 patients treated to date. While liver damage is a known risk and noted in the drug’s safety profile, the severity of this case raises concerns. Sarepta emphasized that this does not represent a new safety signal, maintains the benefit/risk profile of Elevidys remains positive, and has reported the incident to health authorities with plans to update prescribing information. While the perceived clinical benefits of Elevidys have been modest, the sharp market reaction may be an overcorrection given the limited treatment options available for Duchenne muscular dystrophy patients. The safety update also rippled across the Duchenne muscular dystrophy gene therapy space, boosting Regenxbio shares by ~11% on optimism for its RGX-202 program, while weighing on Solid Biosciences, which fell ~8% despite no serious adverse events reported in early trials of its SGT-003 therapy.
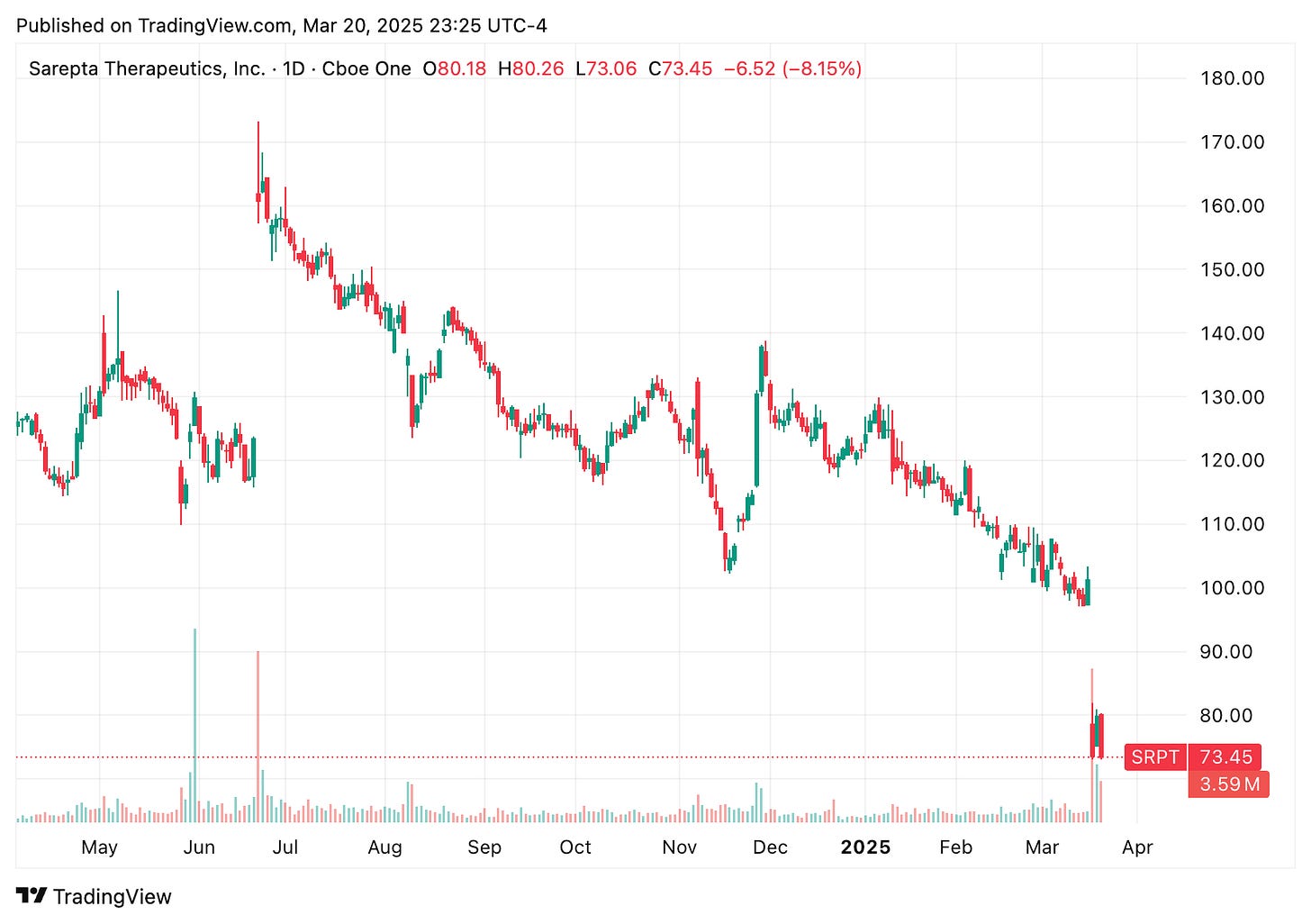
🔹 Later this week, Regenxbio also reported positive interim data from its AFFINITY DUCHENNE trial of RGX-202, showing robust microdystrophin expression (including 122.3% in a 3 year old patient) and continued strong safety with no serious adverse events. The gene therapy remains well tolerated across age groups and is the only next-generation Duchenne therapy enrolling patients under age 4 in the U.S. The phase 3 trial is ongoing, with a Biologics License Application submission targeted for mid-2026. The news further boosted the company’s stock ~4%, which had already risen following Sarepta’s announcement of a patient death linked to treatment for the same disease.
BIOTECH NEWS
🔹 Monte Rosa Therapeutics reported strong progress across its molecular glue degrader (MGD) pipeline. In a phase 1 study, MRT-6160, a VAV1 degrader for immune-mediated diseases, demonstrated over 90% target degradation, significant inhibition of T and B cell activation and cytokine release, and a favorable safety profile, supporting advancement to phase 2 in collaboration with Novartis.
🔹 Alnylam Pharmaceuticals announced that the FDA has approved Amvuttra (vutrisiran) for the treatment of cardiomyopathy in adults, expanding its earlier approval for polyneuropathy. Based on results from the HELIOS-B phase 3 trial, the drug demonstrated significant reductions in cardiovascular death, hospitalizations, and disease progression. Administered quarterly via subcutaneous injection, Amvuttra targets the disease at its genetic source by silencing transthyretin production through RNA interference. Global regulatory reviews are ongoing, and Alnylam expects broad insurance coverage with most patients paying $0 out of pocket.
🔹 On Mar. 20, Elevation Oncology announced it will discontinue development of its cancer drug EO-3021 after phase 1 results showed limited efficacy, with only one confirmed complete response among 36 patients. The company will now shift focus to its HER3-targeting ADC, EO-1022, with an IND filing planned for 2026. As part of the pivot, Elevation is cutting approximately 70% of its workforce and evaluating strategic options. Despite strong safety data, the disappointing trial outcome led to a stock drop of over 40% as investors reacted to the company’s narrowed pipeline and restructuring plans.
🔹 After canceling a routine public meeting of its Vaccines and Related Biological Products Advisory Committee (VRBPAC), the FDA announced its flu vaccine strain recommendations for the 2025-2026 season on Mar. 14, following a closed-door meeting with other government agencies. The unannounced meeting, attended by officials from the FDA, CDC, and Department of Defense, was held in place of the canceled VRBPAC meeting and ended early. Experts voiced concerns about the lack of transparency, though the FDA stated that the selected strains closely align with past recommendations and those of the WHO, ensuring no anticipated impact on vaccine availability. The decision drew criticism during the confirmation hearing for Martin Makary, Trump’s nominee for FDA Commissioner, who pledged to review the meeting’s cancellation but questioned its necessity. Senate Health Committee Chair Bill Cassidy criticized the move as contradicting promises of transparency from the Biden administration’s health agencies. Makary defended the decision, arguing that flu vaccine strain selection is largely a routine process and downplayed VRBPAC’s role. Despite Trump’s order for the U.S. to withdraw from WHO, U.S. health officials participated in the WHO-led Global Influenza Program meeting to determine vaccine composition.
CLINICAL TRIAL UPDATES
🔹 Hutchmed and Innovent announced that their phase 2/3 FRUSICA-2 trial in China met its primary endpoint, showing that the combination of fruquintinib and sintilimab significantly improved progression-free survival in patients with advanced renal cell carcinoma. The combo also showed benefits in secondary measures like objective response rate and duration of response, supporting plans for regulatory filings in the coming months. Despite missing 2024 revenue expectations, Hutchmed shares rose on the positive trial news, highlighting strong investor optimism around the drug’s potential.
🔹 Acumen Pharmaceuticals announced positive topline results from a phase 1 study evaluating the subcutaneous (SC) formulation of sabirnetug, a monoclonal antibody targeting toxic amyloid beta oligomers, in healthy volunteers. The drugs is currently being evaluated in the ongoing phase 2 ALTITUDE-AD trial for early Alzheimer’s disease. The SC version, administered weekly, was well-tolerated and achieved sufficient systemic exposure to support continued development. Mild injection site reactions were the most common adverse event. The SC formulation, enabled by Halozyme’s Enhaze technology, offers the potential for increased patient convenience compared to intravenous administration.
🔹 Immunovant announced positive results from its phase 3 study of batoclimab in myasthenia gravis (MG) and initial phase 2b data in chronic inflammatory demyelinating polyneuropathy (CIDP), showing that greater IgG reductions led to increased clinical improvements. Both studies confirmed batoclimab’s consistent safety profile. While Immunovant does not currently plan to seek regulatory approval for batoclimab in these indications, the data will inform registrational studies of its next generation FcRn inhibitor, IMVT-1402, which the company aims to position as a best in class therapy.
🔹 Scholar Rock presented new data from its phase 3 SAPPHIRE trial at the 2025 MDA Conference, showing that apitegromab achieved a statistically significant and clinically meaningful improvement in motor function for patients with spinal muscular atrophy (SMA) already receiving SMN-targeted therapies. The treatment led to consistent benefits across subgroups and multiple outcome measures. Apitegromab was well tolerated, with no treatment related serious adverse events reported, reinforcing its potential as a first in class, muscle-targeted therapy for SMA.
🔹 On Mar. 16, Dyne Therapeutics announced new long-term data from its phase 1/2 DELIVER trial showing that DYNE-251, its investigational therapy for Duchenne muscular dystrophy (DMD) amenable to exon 51 skipping, demonstrated unprecedented and sustained functional improvement through 18 months at the selected 20 mg/kg dose. The therapy also continued to show a favorable safety profile, with no new treatment-related serious adverse events reported. The DELIVER registrational expansion cohort is fully enrolled, with data expected in late 2025 and a potential U.S. accelerated approval filing planned for early 2026.
PUBLIC HEALTH SPOTLIGHT
🔹 The U.S. Department of Agriculture announced plans to invest up to $100M in research projects focused on developing therapies and vaccines to combat bird flu. This initiative follows a broader $1B commitment made in February to combat the outbreak and stabilize egg prices. The U.S. bird flu vaccine stockpile currently includes products from GSK, Sanofi, and CSL, while other vaccine developers targeting bird flu include Moderna, Pfizer, and CureVac.
🔹 The White House is considering former Republican Representative and physician Michael Burgess to lead the U.S. CDC, following the withdrawal of vaccine skeptic Dave Weldon’s nomination. Burgess, 74, retired from Congress in January after 22 years and has publicly supported vaccines as safe and effective, emphasizing the importance of addressing vaccine hesitancy during the COVID-19 pandemic. Sources say he is the likely nominee, though no final decision has been made.
🔹 Under HHS Secretary Robert F. Kennedy Jr., experts fear that deregulation of stem cell therapies could lead to a surge in unproven and potentially harmful stem cell clinics. Kennedy recently hosted a closed door roundtable on stem cell deregulation, leaving out key FDA officials while including proponents of unapproved treatments. Critics warn that weakening FDA oversight could legitimize questionable practices, reduce incentives for rigorous clinical trials, and expand a risky direct-to-consumer market. If the FDA scales back enforcement or reclassifies stem cell products, experts say both patients and researchers may face increased harm, misinformation, and regulatory gaps.
ON THE HORIZON
🔹 March 2025 FDA PDUFAs:
Mar. 18: NT-501, developed by Neurotech Pharmaceuticals, is a treatment designed for Macular Telangiectasia Type 2 (MacTel), a rare neurodegenerative eye disease that causes progressive central vision loss. NT-501 uses a cell-based delivery system, known as Encapsulated Cell Therapy, to provide sustained release of ciliary neurotrophic factor, a neuroprotective protein that supports photoreceptor survival and slows retinal degeneration. APPROVED ✅
Mar. 23: Vutrisiran is an investigational RNAi therapeutic being developed by Alnylam for the treatment of ATTR amyloidosis with cardiomyopathy (ATTR-CM), a serious and progressive disease characterized by misfolded transthyretin proteins that accumulate in tissues such as the heart. This therapy works by reducing both mutant and wild-type transthyretin (TTR) proteins, addressing the underlying cause of ATTR amyloidosis. If approved, vutrisiran would be the first therapy available to treat both the polyneuropathy and cardiomyopathy manifestations of this disease. APPROVED ✅
Mar. 26: Gepotidacin is a novel, oral antibiotic developed by GSK that is currently under investigation for the treatment of uncomplicated urinary tract infections (UTIs) and gonorrhea.
Mar. 27: Milestone’s Etripamil nasal spray, with a PDUFA date of Mar. 27, is designed to be self-administered as a nasal spray to rapidly treat episodes of paroxysmal supraventricular tachycardia (PSVT), a type of abnormal heart rhythm, allowing patients to manage their symptoms at home without immediate medical intervention; it works by blocking calcium channels in the heart to slow down the rapid heart rate.
In my January 2025 deep dive where I discussed some of the most exciting drugs that could potentially receive FDA approval this year, I covered both gepotidacin and etripamil:
Mar. 28: Sanofi’s Fitusiran is an investigational therapy for hemophilia A or B, used to prevent bleeding in patients, including those with inhibitors. It works by lowering antithrombin to enhance blood clotting, and is administered subcutaneously, potentially reducing treatment frequency to just six doses a year.
🔹 April 2025 FDA PDUFAs:
Apr. 2: Reproxalap is an investigational drug developed by Aldeyra Therapeutics for the treatment of dry eye disease. It functions as a small-molecule modulator of reactive aldehyde species, which are elevated in ocular and systemic inflammatory diseases.
Apr. 3: Cabozantinib, marketed by Exelixis under the brand name Cabometyx, is an oral tyrosine kinase inhibitor approved for treating various cancers, including advanced renal cell carcinoma, hepatocellular carcinoma, and differentiated thyroid cancer.
Apr. 3: Amgen's (Horizon Therapeutics) Inebilizumab (Uplizna) for IgG4-related disease (supplemental BLA for the CD19-targeted mAb’s new use).
Apr. 18: Dupilumab (Dupixent), developed by Regeneron and Sanofi, is an anti-inflammatory biologic already approved for several conditions like atopic dermatitis, asthma, and eosinophilic esophagitis. The FDA is currently reviewing two supplemental applications: one for bullous pemphigoid (PDUFA date: June 20, 2025) and another for chronic spontaneous urticaria (PDUFA date: April 18, 2025), which could further expand its use.
Apr. 26: Telix Pharma’s TLX101-CDx (Pixclara), imaging agent for recurrent glioma (new NDA for F-18 FET diagnostic tracer) awaiting its decision on Apr. 26.
Apr. 29: Elamipretide is an investigational peptide developed by Stealth BioTherapeutics, designed to target mitochondrial dysfunction by binding to cardiolipin in the inner mitochondrial membrane, thereby enhancing mitochondrial function. It is primarily being developed for the treatment of Barth syndrome, an ultra-rare genetic disorder characterized by cardiac abnormalities, muscle weakness, and reduced life expectancy.
Apr. 29: J&J’s Nipocalimab for generalized Myasthenia Gravis (gMG) in adults who are anti-AChR, anti-MuSK or anti-LRP4 antibody-positive (original BLA, anti-FcRn monoclonal antibody). The product has orphan designation and priority review.
Apr. 30: Dihydroergotamine (DHE) is used for the acute treatment of migraine with or without aura in adults. Satsuma Pharmaceuticals is developing STS101, a novel DHE nasal powder formulation designed for fast, easy self-administration and rapid absorption.
Have a great rest of your week and thanks for reading Biotech Blueprint.
👩🏻💻 BIOTECH BLUEPRINT CONSULTING
I provide tailored consulting solutions designed to meet the unique challenges of both established companies and startups. My services span a wide range of strategic and technical needs, including:
Research strategy & grant writing
Scientific communication & medical affairs
Data analysis & interpretation
Biotech/pharma innovation & technology assessment
Startup advisory services
I also provide daily Biotech Blueprint newsletters and custom daily analysis (charts & graphs) for individuals and companies.
BOOK A FREE 30-MINUTE CONSULTATION OR A MEET & GREET below.
DISCLAIMER: This content is for informational purposes only. It should not be taken as legal, tax, investment, financial, or other advice. The views expressed here are my own and do not reflect the opinions of any company or institution.
DISCLOSURE: I have no business relationships with any company mentioned in this article.