This Week in Biotech #41
Catch up on the latest biotech breakthroughs and upcoming trends (Feb 21-27).
Welcome back to This Week in Biotech by Biotech Blueprint, edition 41.
THIS WEEK’S KEY TAKEAWAYS 🔑
This week’s developments point to a broader reassessment of health priorities by the new administration, with key vaccine planning meetings canceled and ongoing reviews of major contracts, suggesting potential shifts in response capabilities at a time when multiple infectious threats demand attention. Key highlights include:
The FDA’s unexpected cancellation of its March advisory committee meeting on flu strain selection raises concerns about vaccine development timelines amid an already severe influenza season, potentially compromising readiness for 2025-26
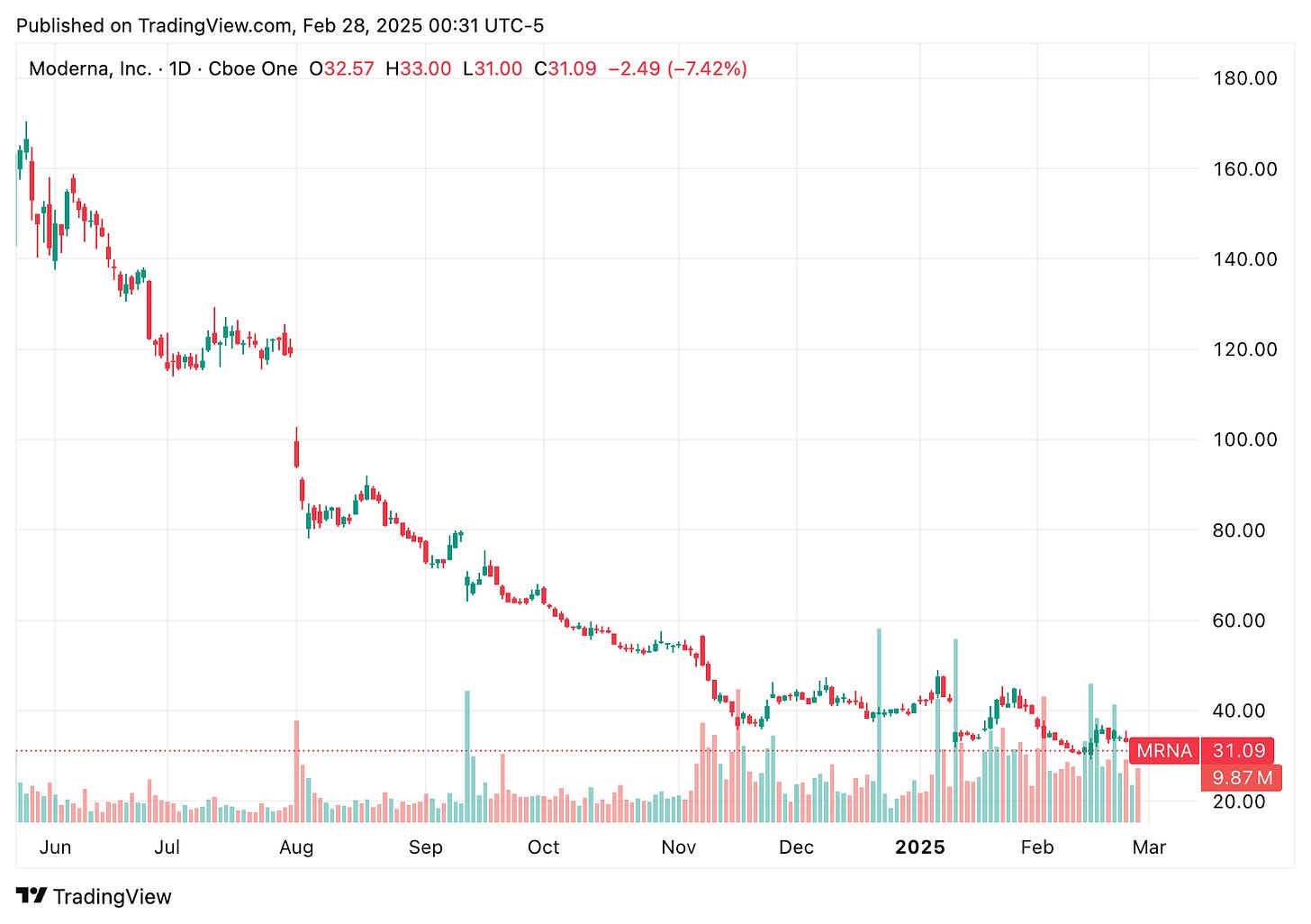
Another potential setback for Moderna: the company faces uncertainty as its $590M bird flu vaccine contract undergoes review by the new administration, highlighting how political transitions can disrupt pharmaceutical development pipelines and public-private health partnerships. This latest development follows a string of challenges for the company, with its stock dropping another 7% yesterday. It feels like there’s a new obstacle every other week for Moderna lately.
The discovery of HKU5-CoV-2, a novel bat coronavirus in China, is as a reminder that viral surveillance remains critical, even as experts caution against unnecessary alarm.
These stories highlight the growing tensions between maintaining scientific integrity in public health decision making and navigating shifting political priorities and economic pressures.
Read the rest of This Week in Biotech #41 below or listen to the AI-generated podcast for more biotech and pharma news. Happy Friday!
🎙️ PODCAST
BIOTECH NEWS
🔹 The chikungunya vaccine (IXCHIQ), manufactured by Valneva, is licensed for adults aged 18+ in the U.S. It was approved by the FDA in Nov. 2023, with the Advisory Committee on Immunization Practices (ACIP) recommending it for travelers and laboratory workers at increased risk of exposure. The CDC is investigating five hospitalizations in individuals aged 65+ for cardiac or neurologic events after vaccination.
🔹 Organovo Holdings has announced that Eli Lilly will acquire its FXR program, including its lead asset, FXR314, which is being developed for inflammatory bowel disease. The acquisition includes all commercial and intellectual property rights for worldwide development of the FXR program. Organovo will receive an upfront payment and additional milestone payments as FXR314 reaches key regulatory and commercial milestones. This partnership allows Lilly to advance FXR314 into phase 2 development. Organovo specializes in using 3D human tissue models for drug development.
🔹 Summit Therapeutics and Pfizer have announced a clinical trial collaboration to evaluate ivonescimab, an investigational PD-1/VEGF bispecific antibody, in combination with several of Pfizer’s antibody-drug conjugates (ADCs) across various solid tumor settings. The goal is to accelerate the development of new cancer treatment options. The trials will assess the safety and anti-tumor activity of ivonescimab in combination with Pfizer’s vedotin-based ADCs in distinct solid tumor settings. Summit will provide ivonescimab, while Pfizer will conduct the trials. The trials are expected to begin mid-2025.
🔹 Guardant Health has sued Natera in California federal court, accusing the company of stealing trade secrets related to cancer-detection blood tests. The lawsuit claims two former Guardant scientists transferred confidential files to Natera before leaving the company. Natera denied the allegations, calling them “baseless.” This case follows a previous $292.5M verdict Guardant won against Natera in November over false advertising. Guardant is seeking to block the former employees from sharing its secrets and is requesting monetary damages. The case is filed in the U.S. District Court for the Northern District of California.
🔹 Mirum Pharmaceuticals’ Ctexli (chenodiol) tablets have received FDA approval for the treatment of cerebrotendinous xanthomatosis (CTX) in adults. Ctexli is the first and only approved treatment for this rare disease. The approval follows positive results from the phase 3 RESTORE study, which showed significant reduction in urine bile alcohols and improved serum cholestanol levels. Ctexli was also granted Orphan Drug exclusivity by the FDA. Ctexli will be available through Mirum’s patient support program, Mirum Access Plus.
🔹 Viking Therapeutics (VKTX) stock surged 12% following renewed takeover speculation, with traders citing a Betaville alert suggesting Pfizer may be interested in acquiring the obesity drug maker. Morgan Stanley is reportedly advising on the deal. Viking, with a market cap of $3.5B, had previously been linked to Eli Lilly in takeover talks last year. The company is developing a dual GLP-1/GIP agonist drug for metabolic disorders like obesity. Viking has also seen growing attention after Roche’s $3.1B acquisition of Carmot Therapeutics in Dec. 2023.
🔹 Gilead’s Seladelpar has received conditional marketing authorization from the European Commission for treating primary biliary cholangitis. It’s the first treatment to show significant improvements in biochemical response, ALP normalization, and pruritus compared to placebo. Cholangitis, a rare liver disease, primarily affects women and can progress to liver failure. Seladelpar has shown anti-inflammatory and anti-pruritic effects, addressing a major unmet need. The approval follows positive phase 3 trial results and comes after FDA and U.K. approvals. Further confirmatory trials are required for continued approval.
🔹 bluebird bio announced it has entered into a definitive agreement to be acquired by Carlyle and SK Capital and become private held biotech, with stockholders set to receive $3.00 per share in cash and a contingent value right of $6.84 per share, subject to sales milestones. Despite the strategic review process and numerous discussions with over 70 potential investors, the company faced significant financial challenges, including the risk of defaulting on loan covenants, making this acquisition the only viable solution. The stock plummeted over 35% following the an
CLINICAL TRIAL UPDATES
🔹 Candel Therapeutics announced positive final survival data from its phase 2 clinical trial of CAN-2409 in patients with borderline resectable pancreatic ductal adenocarcinoma (PDAC). The trial showed that patients treated with CAN-2409, combined with standard therapy, had a significant improvement in overall survival, with a median survival of 31.4 months compared to 12.5 months in the control group. Three patients treated with CAN-2409 survived for 66, 63.6, and 35.8 months post-enrollment, with survival well beyond the expected median for PDAC. The treatment was well-tolerated with no significant toxicities, and the immune response was robust, supporting CAN-2409’s potential as a treatment for PDAC. Candel plans to proceed with larger trials based on these promising results.
🔹 Regeneron Pharmaceuticals has announced positive results from the phase 1/2 CHORD trial of its investigational gene therapy DB-OTO for children with profound genetic hearing loss due to otoferlin (OTOF) gene variants. The trial showed significant improvements in hearing for 10/11 children assessed, including a child who gained near-normal hearing and demonstrated progress in speech and development. The gene therapy was well tolerated, with no serious adverse events related to DB-OTO. DB-OTO has received several designations from the FDA, including Orphan Drug and Fast Track. The trial is ongoing across multiple global sites.
PUBLIC HEALTH SPOTLIGHT
🔹 The FDA has canceled its Mar. 13 meeting of the Vaccines and Related Biological Products Advisory Committee (VRBPAC), which was scheduled to recommend flu strains for the 2025-26 flu shot in the Northern Hemisphere. The meeting’s cancellation raises concerns about the timing and availability of next season’s flu vaccine. The FDA confirmed the cancelation but assured that vaccines would still be available on time. The decision comes amid a severe flu season in the U.S. and follows the postponement of a CDC meeting and a stop-work order on a COVID vaccine contract, prompting fears about undermining vaccine policies.
🔹 Moderna’s stock dropped by 6% yesteday following reports that the Trump administration is reevaluating a $590M bird flu vaccine contract awarded to the company by the Biden administration. The review is part of efforts to assess spending on mRNA vaccines, including Moderna’s COVID-19 vaccine. The contract was granted during Biden’s tenure amid concerns that the Trump administration might reduce funding for vaccine makers. Moderna’s planned late-stage study for its pandemic influenza vaccine could be affected if the funding is withdrawn. Meanwhile, bird flu has significantly impacted U.S. poultry, leading to rising egg prices.
🔹 The U.S. government will invest up to $1B to combat bird flu and alleviate high egg prices. The funding includes $500M for biosecurity audits on farms and $400M to support farmers who need to cull infected chickens. Since 2022, bird flu has killed 166M chickens, with nearly 1,000 dairy herds and 70 people affected. The USDA is considering using vaccines for chickens but hasn’t yet authorized them due to trade concerns. Additionally, the U.S. will increase egg imports from Turkey to help reduce prices, which have nearly doubled. The U.S. also plans to decrease egg exports to boost domestic supply.
🔹 The recent discovery of a new bat coronavirus, HKU5-CoV-2, has raised concerns, but experts emphasize that it does not pose an immediate public health threat. The virus, detected in bats in China, can potentially infect human cells, similar to SARS-CoV-2, but has not been shown to infect humans or spread between them. Despite some alarm due to its connection to the Wuhan Institute of Virology, experts state that the virus is not currently a cause for panic. The CDC and various infectious disease doctors reassured the public, stressing that many viruses are found in animals but do not lead to pandemics. As a result of this news, stocks of vaccine companies like Moderna and Novavax have experienced a rise, reflecting increased investor attention on potential future vaccine development in response to new virus strains.
ON THE HORIZON
🔹 March 2025 FDA PDUFAs:
Mar. 18: NT-501, developed by Neurotech Pharmaceuticals, is a treatment designed for Macular Telangiectasia Type 2 (MacTel), a rare neurodegenerative eye disease that causes progressive central vision loss. NT-501 uses a cell-based delivery system, known as Encapsulated Cell Therapy, to provide sustained release of ciliary neurotrophic factor, a neuroprotective protein that supports photoreceptor survival and slows retinal degeneration.
Mar. 23: Vutrisiran is an investigational RNAi therapeutic being developed by Alnylam for the treatment of ATTR amyloidosis with cardiomyopathy (ATTR-CM), a serious and progressive disease characterized by misfolded transthyretin proteins that accumulate in tissues such as the heart. This therapy works by reducing both mutant and wild-type transthyretin (TTR) proteins, addressing the underlying cause of ATTR amyloidosis. If approved, vutrisiran would be the first therapy available to treat both the polyneuropathy and cardiomyopathy manifestations of this disease.
Mar. 26: Gepotidacin is a novel, oral antibiotic developed by GSK that is currently under investigation for the treatment of uncomplicated urinary tract infections (UTIs) and gonorrhea.
Mar. 27: Milestone’s Etripamil nasal spray, with a PDUFA date of Mar. 27, is designed to be self-administered as a nasal spray to rapidly treat episodes of paroxysmal supraventricular tachycardia (PSVT), a type of abnormal heart rhythm, allowing patients to manage their symptoms at home without immediate medical intervention; it works by blocking calcium channels in the heart to slow down the rapid heart rate.
In one of my January 2025 deep dives where I discussed some of the most exciting drugs that could potentially receive FDA approval this year, I covered both gepotidacin and etripamil:
Mar. 28: Sanofi’s Fitusiran is an investigational therapy for hemophilia A or B, used to prevent bleeding in patients, including those with inhibitors. It works by lowering antithrombin to enhance blood clotting, and is administered subcutaneously, potentially reducing treatment frequency to just six doses a year.
Have a great rest of your week and thanks for reading Biotech Blueprint.
👩🏻💻 BIOTECH BLUEPRINT CONSULTING
I provide tailored consulting solutions designed to meet the unique challenges of both established companies and startups. My services span a wide range of strategic and technical needs, including:
Research strategy & grant writing
Scientific communication & medical affairs
Data analysis & interpretation
Biotech/pharma innovation & technology assessment
Startup advisory services
I also provide daily Biotech Blueprint newsletters and custom daily analysis (charts & graphs) for individuals and companies.
BOOK A FREE 30-MINUTE CONSULTATION OR A MEET & GREET below.
DISCLAIMER: This content is for informational purposes only. It should not be taken as legal, tax, investment, financial, or other advice. The views expressed here are my own and do not reflect the opinions of any company or institution.
DISCLOSURE: I have no business relationships with any company mentioned in this article.