This Week in Biotech #15
Catch up on the latest biotech breakthroughs and upcoming trends (Sept 27-Oct 1).
Welcome to the Wednesday edition of This Week in Biotech by Biotech Blueprint!
MARKET UPDATES
🔹 On Oct. 1, Gritstone bio announced phase 2 data for its individualized neoantigen immunotherapy, GRANITE, in patients with colorectal cancer. Key findings include a 21% relative risk reduction in progression or death compared to standard treatment, with a 38% reduction in a subgroup with lower levels of circulating tumor DNA. GRANITE was well-tolerated with no treatment discontinuations. Despite the encouraging data, Gritstone’s stock plummeted by 62% following the announcement. The company is exploring strategic options for advancing the therapy.
🔹 On Sept. 30, Prime Medicine (PRME) announced a strategic collaboration and license agreement with Bristol Myers Squibb to develop and commercialize ex vivo T-cell therapies using Prime Medicine’s advanced gene-editing technology. The deal includes a $110M upfront payment to Prime Medicine, with potential milestone payments exceeding $3.5B. Following the announcement, Prime’s stock rose by 15% on Monday.
🔹 On Sept. 27, Cantor Fitzgerald initiated coverage of Amgen (AMGN) with an Overweight rating and a $405 price target, highlighting the significant opportunity presented by their obesity drug candidate, MariTide. The firm views MariTide as a potential $15B-$30B revenue driver, with success in phase 2 expected to de-risk the program and dramatically boost Amgen’s growth outlook. Even without the obesity program, Cantor expects Amgen’s new product portfolio to drive revenue growth through the end of the decade.
Read my latest deep dive into GLP-1 drugs by Amgen and other companies here:
🔹 On Sept. 27, Citi downgraded Summit Therapeutics (SMMT) from a Buy to a Neutral rating while raising the price target from $19 to $23. This decision follows a dramatic 670% increase in the stock price since May, reaching a market capitalization of around $16B. Citi’s downgrade is attributed to an assessment of the stock’s current valuation post the HARMONi-2 study results. While the data for Ivonescimab, a treatment for non-small cell lung cancer (NSCLC), is promising, with an 85% probability of success and projected sales of over $6.5B by 2035, analysts concluded that the stock’s valuation has surpassed realistic expectations.
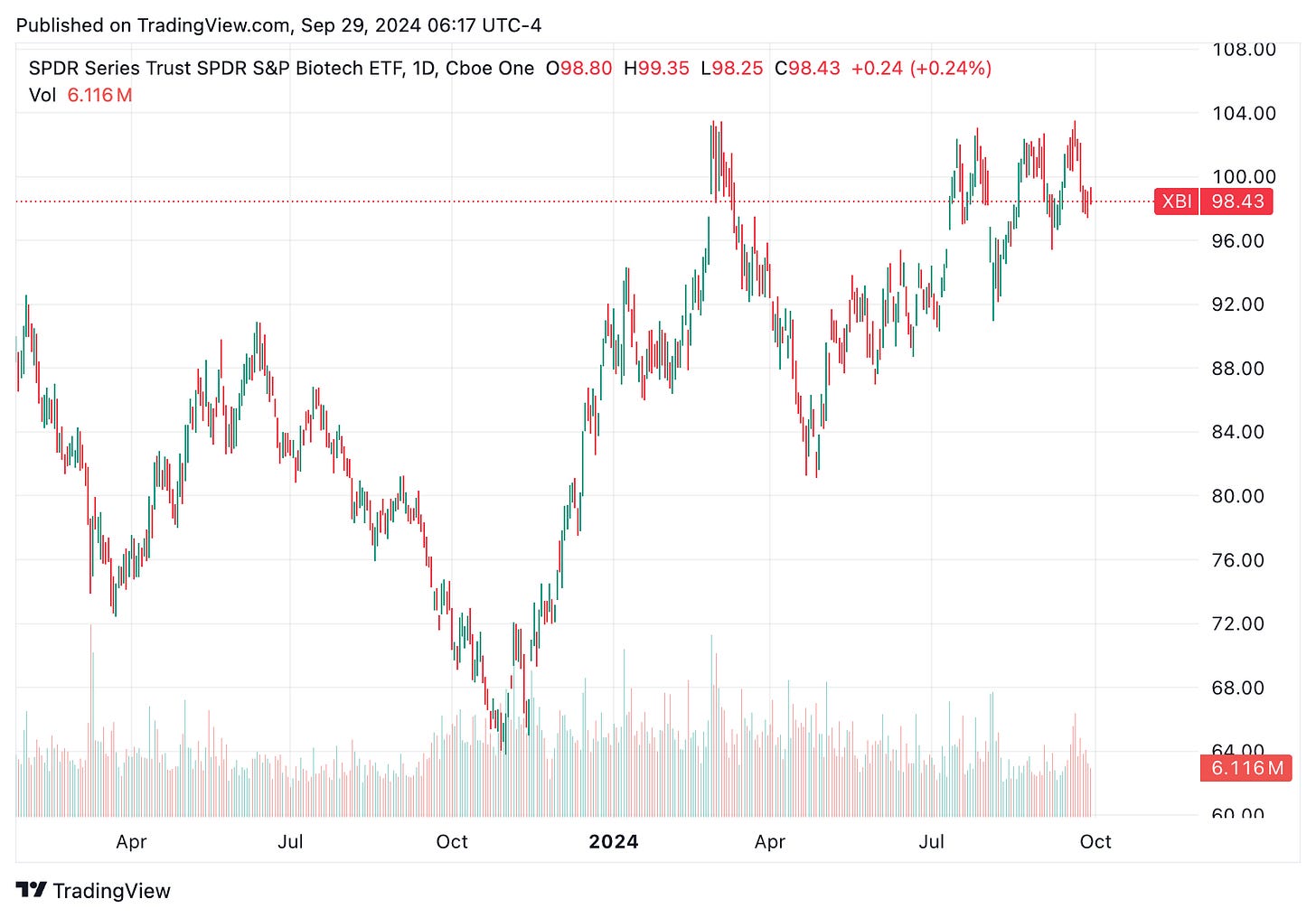
🔹 Biotech IPOs are rebounding as easing interest rates reduce funding constraints that have plagued the sector. Biotech companies were hit hard when the U.S. Federal Reserve raised interest rates 11 times between March 2022 and July 2023, reaching 5.25%–5.5%. This led to a sharp decline in IPOs, with only 19 biotech companies going public in 2023 compared to >100 in 2021, and most experiencing falling stock prices. However, signs of recovery emerged before the Fed’s recent rate cut to 4.75%–5%, with the SPDR S&P Biotech ETF (XBI) gaining 53% since October 2023. IPO activity is also picking up, with 20 biotechs going public this year, already surpassing last year’s total.
BIOTECH NEWS
🔹 On Oct. 1, Amneal Pharmaceuticals and Metsera announced a collaboration to develop and supply obesity and metabolic disease drugs. Under the agreement, Amneal will build manufacturing facilities to support Metsera’s novel injectable and oral therapies, including GLP-1 and amylin receptor agonists. The collaboration is set to accelerate product development and future commercial launches, starting with the GLP-1 receptor agonist, MET-097, which showed promising phase 1 results.
🔹 On Oct. 1, Shattuck Labs announced a discontinuation of its SL-172154 program after interim clinical data showed modest improvement in overall survival for TP53 mutant acute myeloid leukemia and myelodysplastic syndromes. This strategic shift is accompanied by a 40% workforce reduction, aimed at extending the company’s cash runway into 2027.
🔹 On Sept. 30, IGM Biosciences announced a strategic shift to focus solely on autoimmune diseases, reducing its investment in oncology, including aplitabart. Key leadership changes were made, with CEO Fred Schwarzer, CSO Bruce Keyt, and CMO Chris Takimoto all stepping down. Mary Beth Harler, M.D., was appointed as the new CEO.
🔹 On Sept. 27, AbbVie submitted a biologics license application to the FDA for Telisotuzumab Vedotin (Teliso-V), an investigational antibody-drug conjugate for previously treated nonsquamous non-small cell lung cancer (NSCLC) with c-Met protein overexpression. This application seeks accelerated approval, backed by data from the Phase 2 LUMINOSITY trial.
🔹 On Sept. 27, Sanofi/Regeneron’s Dupixent (dupilumab) was approved by the FDA as the first biologic treatment for adults with inadequately controlled chronic obstructive pulmonary disease (COPD), affecting approximately 300k people in the U.S. The approval follows successful phase 3 trials where the drug improved lung function, and enhanced quality of life compared to placebo. Dupixent is already widely prescribed for asthma and atopic dermatitis.
🔹 Bavarian Nordic signed an agreement with UNICEF to supply 1M doses of its JYNNEOS (MVA-BN) mpox vaccine for African countries affected by the mpox outbreak, marking the first award under UNICEF’s emergency tender.
CLINICAL TRIAL UPDATES
🔹 On Sept. 30, Neurocrine Biosciences presented interim results from its ongoing study showing sustained improvements in chorea (uncontrollable dance-like movements) associated with Huntington’s disease with Ingrezza (valbenazine). The improvements were consistent regardless of antipsychotic use. Key findings demonstrated a reduction in chorea symptoms as early as week 2, with further sustained improvements over time. By week 104, about 75% of participants reported improvement. The most common adverse events included somnolence, falls, and fatigue, aligning with previous findings.
🔹 On Sept. 30, Kezar Life Sciences paused patient enrollment and dosing in its phase 2b PALIZADE trial of zetomipzomib for lupus nephritis after four deaths in patients from the Philippines and Argentina, with a concerning pattern of symptoms related to dosing. The company plans to assess the safety data and consult with regulatory authorities before determining the trial’s future.
🔹 On Sept. 30, Rivus Pharmaceuticals announced promising new data from the phase 2a HuMAIN trial, showing that their novel therapy HU6 led to significant fat-selective weight loss in patients with obesity-related heart failure. Patients experienced reductions in visceral fat without loss of lean body mass, improvements in blood pressure, and reductions in key cardiovascular disease markers. HU6 was well tolerated.
🔹 On Sept. 28, Enliven Therapeutics announced positive data from its phase 1 trial of ELVN-001, a novel BCR-ABL kinase inhibitor for chronic myeloid leukemia. The updated data showed a 44% major molecular response rate by 24 weeks. The trial enrolled 39 heavily pretreated CML patients, and ELVN-001 was well-tolerated with no dose reductions or serious adverse events.
🔹❗On Sept. 27, J&J and Legend Biotech announced significant long-term results from the phase 3 trial showing that Carvykti, a CAR-T cell therapy, dramatically improves overall survival in patients with relapsed or lenalidomide-refractory multiple myeloma. Compared to standard therapies, Carvykti reduced the risk of death by 45%. Overall survival rates at 30 months were 76% for Carvykti patients, compared to 64% for those receiving standard treatments. The safety profile was consistent with previous analyses, with common side effects including cytopenia and infections.
🔹 On Sept. 27, Cereno Scientific announced positive topline results from its phase 2a trial of CS1 in treating pulmonary hypertension. CS1 showed a significant positive impact on key clinical parameters and the trial successfully met its primary endpoint of safety and tolerability, with no serious adverse effects reported.
🔹 On Sept. 27, Poseida Therapeutics reported positive phase 1 results for its allogeneic CAR-T therapy, P-BCMA-ALLO1, in relapsed/refractory multiple myeloma. Tthe therapy achieved a 91% overall response rate. Notably, there were no dose-limiting toxicities with no instances of graft-vs-host disease. P-BCMA-ALLO1, which recently received FDA Regenerative Medicine Advanced Therapy designation, is being evaluated in a phase 1/1b trial, with promising efficacy and safety data in heavily pretreated patients.
Happy Wednesday and thanks for reading Biotech Blueprint!
DISCLAIMER: This content is for informational purposes only. It should not be taken as legal, tax, investment, financial, or other advice. The views expressed here are my own and do not reflect the opinions of any company or institution.
DISCLOSURE: I have no business relationships with any company mentioned in this article.





