This Week in Biotech #14
Catch up on the latest biotech breakthroughs and upcoming trends (Sept 25-26).
Welcome to the Friday edition of This Week in Biotech by Biotech Blueprint!
An important development this week is the FDA’s approval of BMS’s Cobenfy (KarXT), a breakthrough that has the potential to transform the treatment landscape for schizophrenia. For more details, check out the BIOTECH NEWS section below.
MARKET UPDATES
🔹 On Sept. 26, Travere Therapeutics announced a pause in enrollment for the phase 3 HARMONY Study of pegtibatinase, a treatment for classical homocystinuria because the “desired drug substance profile was not met during the recent scale-up process.” Travere anticipates that enrollment may resume in 2026, with associated investments delayed beyond 2025. The company shares fell 5% premarket Friday.
🔹 On Sept. 26, AI biotech company Evaxion Biotech announced an expanded collaboration with Merck for two AI-designed preclinical vaccine candidates, EVX-B2 (for Gonorrhea) and EVX-B3 (targeting an undisclosed infectious agent). Following the announcement, Evaxion’s stock surged by 17%. The agreement grants Merck the option to exclusively license these candidates, providing Evaxion with a $3.2M upfront payment and up to $10M by 2025. The deal could potentially lead to $592M in milestone payments per product, highlighting the value of Evaxion’s AI-Immunology platform and advancing its vaccine pipeline.
🔹 Emergent BioSolutions (EBS) has secured $67.4M in additional orders for TEMBEXA (brincidofovir) and $400M in ACAM2000 vaccine orders to bolster U.S. national preparedness against smallpox and mpox. Read more about these developments in the BIOTECH NEWS section. Following this announcement, the company’s stock soared nearly 23%.
🔹 On Sept. 25, 2seventy bio (TSVT) and Bristol Myers Squibb announced the discontinuation of enrollment in the phase 3 KarMMa-9 study for Abecma in newly diagnosed multiple myeloma patients. This led to a brief 16% drop in 2seventy bio’s stock price which has since recovered.
🔹 Humacyte’s (HUMA) stock dropped nearly 8% in premarket trading on Sept. 25 after announcing plans to sell up to $50M in shares to Lincoln Park Capital. Proceeds will support product development, potential commercial launch of its acellular tissue-engineered vessel, and general corporate purposes.
🔹 Amgen’s (AMGN) stock dipped 5% on Sept. 26 following the release of phase 3 data for two drugs, Uplizna and rocatinlimab. The Uplizna trials showed significant improvements in generalized myasthenia gravis and reduced flare risks in IgG4-related disease. Rocatinlimab also showed statistically significant results in treating eczema. Despite the positive data, shares slipped due to Sanofi’s Dupixent and AbbVie’s Rinvoq being the leading treatment options for eczema in the market. While Wells Fargo expressed concerns that Amgen’s rocatinlimab may not be competitive against Dupixent and Rinvoq for eczema, Goldman Sachs took a more optimistic view, highlighting rocatinlimab’s commercial potential due to its safety, tolerability, and convenient dosing.
BIOTECH NEWS
🔹❗ On Sept. 26, the FDA approved COBENFY (KarTX), xanomeline and trospium chloride, a first-in-class muscarinic agonist developed by Bristol Myers Squibb and Karuna, for the treatment of schizophrenia in adults. This represents the first new pharmacological approach for schizophrenia in decades, targeting M1 and M4 receptors in the brain without blocking dopamine D2 receptors, which differentiates it from traditional therapies. The approval is based on data from the EMERGENT clinical program, which demonstrated significant reductions in schizophrenia symptoms.
The drug offers a novel mechanism of action, addressing the complex symptoms of schizophrenia, which include positive symptoms (hallucinations, delusions), negative symptoms (lack of motivation, emotional expression), and cognitive dysfunction.
Cobenfy showed statistically significant improvements in trials, with a reduction of symptoms. The trials also showed that the drug was generally well tolerated, with common side effects like nausea, constipation, and vomiting. This approval provides a new option for managing schizophrenia, a condition where up to 60% of patients experience poor improvement or side effects with current treatments.
🔹 On Sept. 26, Pfizer voluntarily withdrew Oxbryta (voxelotor), a treatment for sickle cell disease, from all global markets following concerns about the drug’s safety. The decision comes after clinical data indicated an imbalance in vaso-occlusive crises and fatal events associated with its use, which are painful complications of sickle cell anemia. Oxbryta, which Pfizer acquired through a $5.4B purchase of Global Blood Therapeutics in 2022, had generated $92M in sales in Q2 2024. The European Medicines Agency (EMA) has recommended suspending Oxbryta’s approval pending further review after data showed a higher incidence of deaths among Oxbryta users compared to placebo in clinical trials.
🔹 On Sept. 26, Sage Therapeutics announced that Biogen has terminated its collaboration on the SAGE-324 program following negative phase 2 trial results for treating essential tremor. Sage will regain full ownership of the asset by February 2025 and may explore other uses for SAGE-324. Despite this, Sage and Biogen will continue their partnership on ZURZUVAE (zuranolone), the FDA-approved oral treatment for postpartum depression.
🔹 On Sept. 26, PTC Therapeutics announced that the FDA has granted fast track designation to its PTC518 program for treating Huntington’s disease. PTC518, an orally administered small molecule, aims to reduce the production of the mutant Huntingtin protein, which causes neuron damage in HD. The PIVOT-HD study showed promising results, including a 43% reduction of mutant Huntingtin protein in blood cells after 12 months and favorable effects on disease progression markers.
🔹 On Sept. 26, Emergent BioSolutions announced securing $67.4M in additional orders for TEMBEXA (brincidofovir), an antiviral for smallpox treatment, under an existing 10-year contract. TEMBEXA is critical for smallpox defense but carries warnings about serious side effects, including liver issues, gastrointestinal problems, and embryo-fetal toxicity.
🔹 Additionally, on Sept. 25, Emergent secured approximately $400M in orders for 2024 and 2025 to support smallpox and mpox preparedness. This includes $185M+ in orders for the ACAM2000 vaccine and CNJ-016 (VIGIV) immune globulin. ACAM2000, recently updated for mpox prevention, has also been donated for African response efforts.
🔹 On Sept. 25, Heron Therapeutics received FDA approval for ZYNRELEF Vial Access Needle (VAN), which is expected to simplify aseptic preparation and significantly reduce the withdrawal time for ZYNRELEF. Set to launch in Q4 2024, the VAN aims to enhance the safe use and adoption of ZYNRELEF, a dual-acting local anesthetic that combines bupivacaine and meloxicam for effective postoperative pain management, thereby improving patient recovery following surgery.
CLINICAL TRIAL UPDATES
🔹 On Sept. 26, Roche announced positive results from its phase 3 REGENCY study, showing that Gazyva/Gazyvaro (obinutuzumab), combined with standard therapy, significantly improved outcomes for patients with lupus nephritis, a severe autoimmune condition affecting 1.7 million people globally, compared to standard therapy alone. The drug, targeting disease-causing B cells, demonstrated a favorable safety profile with no new safety signals. Roche is working with regulators to expedite the drug’s availability.
🔹 On Sept. 26, AbbVie announced positive results from its phase 3 TEMPO-1 trial, evaluating tavapadon for early Parkinson’s disease. Tavapadon significantly improved motor and daily living functions at week 26 compared to placebo. The drug also improved patients’ motor aspects of daily living. Tavapadon is a dopamine receptor partial agonist.
🔹 On Sept. 25, Celldex Therapeutics presented encouraging 52-week results from its phase 2 trial of barzolvolimab for chronic spontaneous urticaria at EADV 2024. The study showed that 71% of patients achieved complete symptom relief, with improvements noted as early as the first week and sustained through the year. While the company reported that adverse events were mild and manageable, the discontinuation rate was rather high. Phase 3 trials are already underway to further assess its efficacy.
🔹 On Sept. 25, Merck announced that its phase 3 KEYFORM-007 trial, assessing the combo of anti-LAG-3 (favezelimab) and Keytruda, did not meet its primary endpoint of improving overall survival in previously treated patients with PD-L1+ colorectal cancer.
SCIENCE SPOTLIGHT
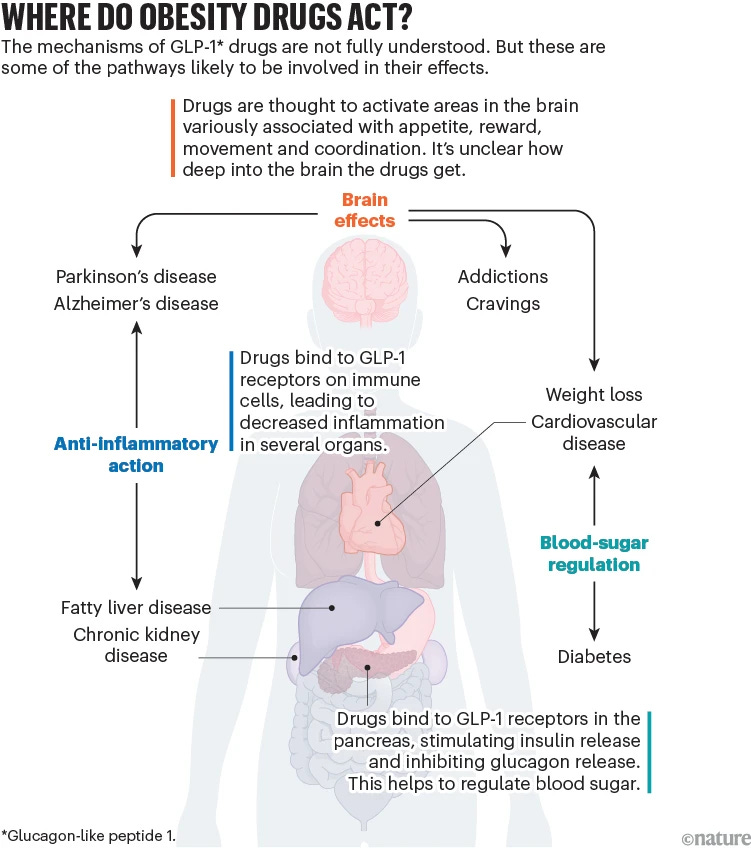
🔹 I had intended to write an article about why GLP-1 obesity drugs seem to address so many health issues beyond just weight loss. But an insightful article published in Nature news on Sept. 25 covered this topic nicely. Here’s the rundown:
GLP-1 drugs are now being investigated for their effects on conditions ranging from addiction to neurodegenerative diseases. At the NIH, for instance, studies are exploring whether GLP-1 drugs can reduce alcohol cravings. Early evidence from animal studies and health records suggests that these medications might dampen various cravings, including those for alcohol and tobacco.
The benefits of GLP-1 drugs extend beyond addiction. They have been shown to reduce risks associated with cardiovascular disease, chronic kidney disease, and even conditions like sleep apnea and Parkinson’s disease. With hundreds of clinical trials currently examining these drugs’ effectiveness across multiple health issues, including fatty liver disease, Alzheimer’s, and HIV complications, scientists believe they might act as potential treatments for a broad spectrum of disorders.
However, understanding the mechanisms at play remains a challenge. In some cases, weight loss might explain the drugs’ benefits, while in others, different pathways, such as anti-inflammatory effects and neural impacts, seem to be involved. One key feature of GLP-1 drugs is their ability to activate GLP-1 receptors not only in the gut but also in the brain, influencing appetite control, mood regulation, and reward systems. This dual action may contribute to their effectiveness in addressing cravings and regulating behaviors associated with addiction.
Importantly, researchers urge caution in promoting these drugs as universal solutions, emphasizing the need for further investigation into long-term effects and appropriate patient selection, especially for those without obesity.
They also included this helpful figure showing where do GLP-1 drugs act in the body:
Here is my latest deep dive if you want to read more about companies that currently have promising GLP-1 drugs in clinical trials:
The expanding landscape of GLP-1 drugs: What's next in the race for new treatments?
In the last article on glucagon-like peptide-1 (GLP-1) receptor agonists, I discussed the soaring demand for drugs like Ozempic, Wegovy, and Zepbound and how they work in the human body. Initially developed for type 2 diabetes, these medications have gained a ton of attention for their weight loss benefits, leading to supply shortages and a surge in dem…
ON THE HORIZON
🔹 October is almost here! Here are October 2024 PDUFAs:
Oct. 2: ARS Pharmaceuticals’ neffy (epinephrine nasal spray) for the treatment of type I allergic reactions including anaphylaxis.
Oct. 4: Biofrontera’s Ameluz (supplemental new drug application to increase treatment dosing) for actinic keratoses.
Oct. 8: Zealand Pharma’s dasiglucagon for treatment of congenital hyperinsulinism.
Oct. 8: Bristol Myers Squibb’s two supplemental biologics applications for Opdivo (nivolumab): Opdivo with chemotherapy followed by surgery and adjuvant Opdivo for the perioperative treatment of resectable stage IIA to IIIB non-small cell lung cancer (NSCLC).
Oct. 17: Amgen’s Lumakras/Lumykras for treatment of metastatic colorectal cancer.
Oct 21: Camurus’ octreotide SC depot (CAM2029) for acromegaly (pituitary gland hormonal disorder).
Happy Friday and thanks for reading Biotech Blueprint!
DISCLAIMER: This content is for informational purposes only. It should not be taken as legal, tax, investment, financial, or other advice. The views expressed here are my own and do not reflect the opinions of any company or institution.
DISCLOSURE: I have no business relationships with any company mentioned in this article.