This Week in Biotech #10
Catch up on the latest biotech breakthroughs and upcoming trends (Sept 10-13).
Welcome to This Week in Biotech by Biotech Blueprint!
MARKET UPDATES
🔹 Fulcrum Therapeutics’ (FULC) stock plummeted 61% after announcing that its phase 3 REACH trial for losmapimod in treating facioscapulohumeral muscular dystrophy (FSHD) failed to meet its primary and secondary endpoints. Losmapimod showed no significant improvement compared to placebo, leading the company to halt its development.
🔹 Immuneering’s (IMRX) stock surged 45% post-market on Sept. 12 after reporting positive initial phase 2a data for its drug candidate IMM-1-104 in combination with gemcitabine/nab-paclitaxel for first-line pancreatic cancer treatment. The study showed a 40% overall response rate and 80% disease control rate in the first five patients, with one patient experiencing a complete response. The drug has been well-tolerated, and additional data is expected by year-end.
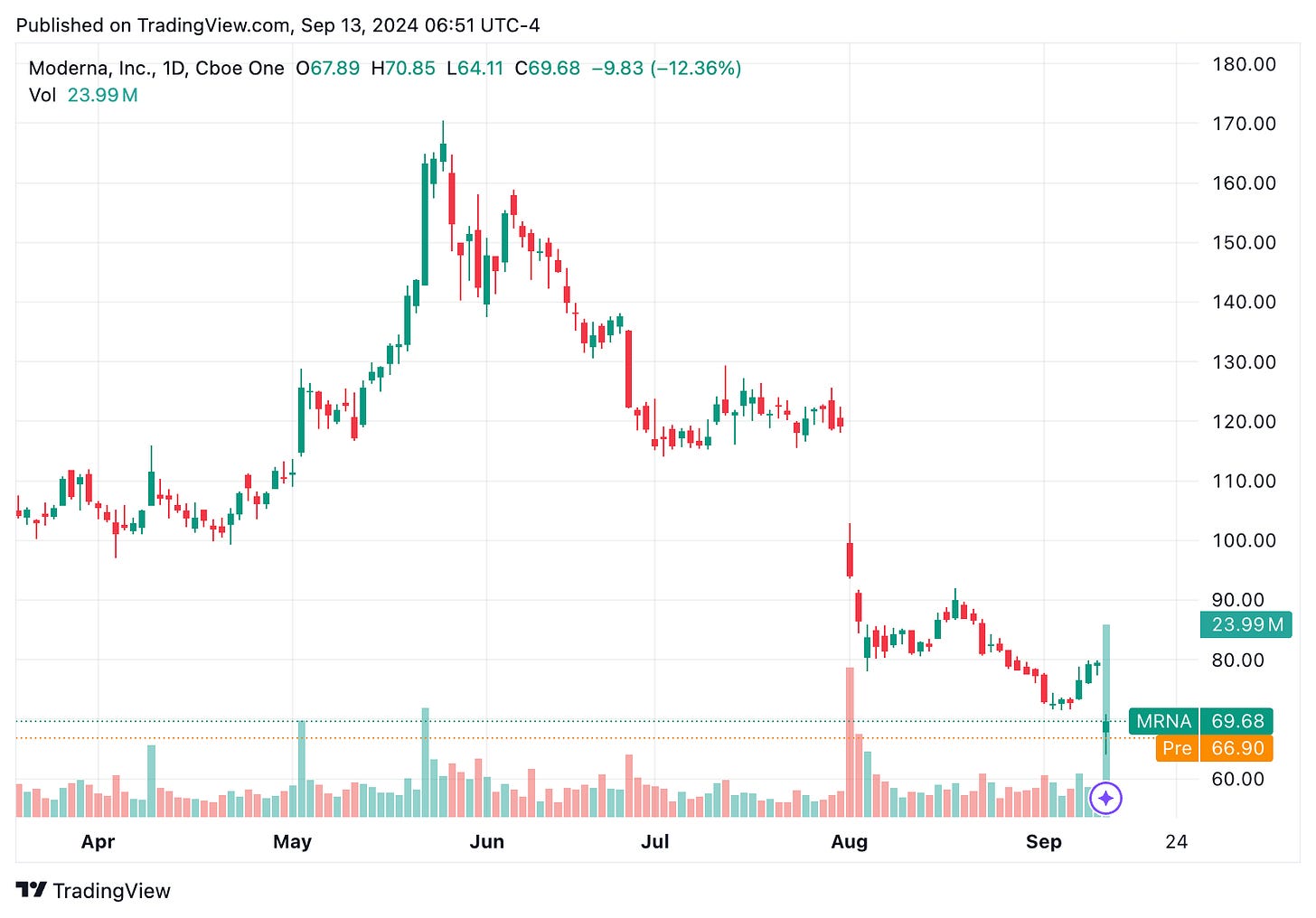
🔹 On Sept. 12, Moderna (MRNA) shares dropped by over 12% after the company announced plans to cut its annual R&D budget by $1.1B starting in 2027. As part of this restructuring, five pipeline programs, including those targeting RSV in infants and KRAS-specific therapy, will be discontinued. The company plans to focus on selective investments and cost efficiencies to reduce R&D expenses while expanding its portfolio into oncology, rare diseases, and vaccines for non-respiratory conditions. Moderna aims to achieve 10 product approvals within the next three years, including its RSV vaccine, mRESVIA, and potential next-gen COVID-19 and flu combination vaccines.
🔹 On Sept. 12, Oncternal Therapeutics’ (ONCT) stock plunged 59% after the company announced it will halt the development of its cancer drugs ONCT-534 and ONCT-808. The decision comes after disappointing phase 1 trial results: ONCT-534 showed dose-limiting toxicities and ONCT-808 had a fatality due to complications. Oncternal will cut costs by reducing its workforce and exploring strategic alternatives like mergers or acquisitions.
🔹 On Sept. 12, Monopar Therapeutics (MNPR) announced positive early clinical data from its ongoing phase 1 trial of MNPR-101-Zr, leading to a 65% surge in its stock price. MNPR-101 is a monoclonal antibody aimed at cancers expressing the urokinase plasminogen activator receptor such as triple-negative breast, colorectal, bladder, ovarian, gastric, and pancreatic cancers. In the trial, MNPR-101-Zr, a zirconium-89 labeled version of the antibody, demonstrated high uptake in metastatic tumors when assessed using total-body PET imaging. This tumor-specific uptake supports its potential for future therapeutic use. Monopar plans to initiate a phase 1 clinical trial of MNPR-101-Lu, a therapeutic version, in late 2024. The company will present additional data at the European Association of Nuclear Medicine conference in October 2024.
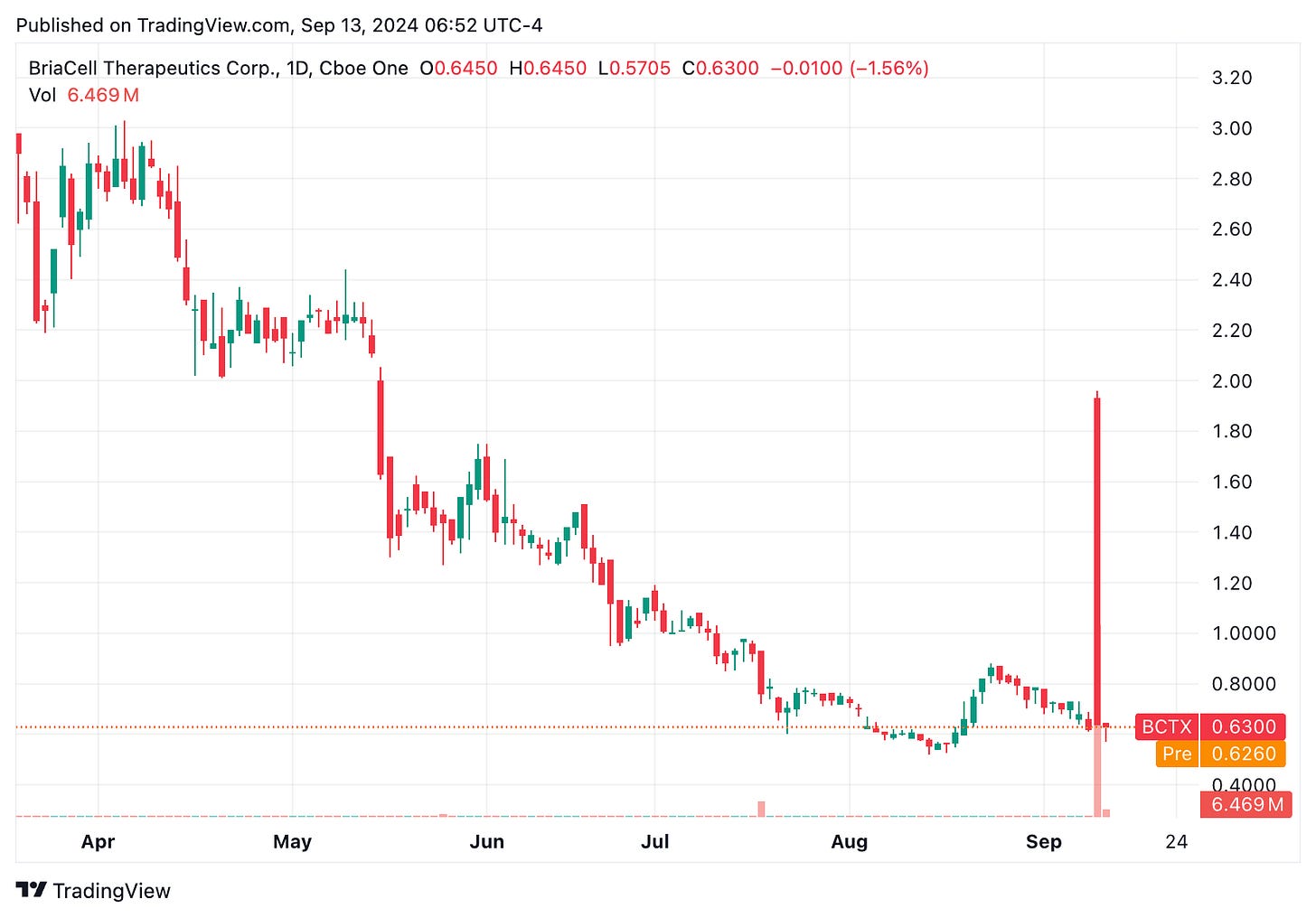
🔹 Shares of BriaCell Therapeutics (BCTX) initially surged 150% on Sept. 11 following the release of positive phase 2 data for its metastatic breast cancer therapy, Bria-IMT. However, the stock quickly pulled back to before the announcement. The study reported a median overall survival of 15.6 months for patients treated with Bria-IMT in combination with an immune checkpoint inhibitor, compared to the 6.7 to 9.3 months typically seen in similar patients. BriaCell noted that this formulation is also being used in an ongoing phase 3 trial.
🔹 Shares of Viking Therapeutics (VKTX) jumped over 11% on Sept. 11 after J.P. Morgan initiated coverage with an “Overweight” rating and an $80 price target, citing optimism around its oral obesity therapy, VK2735. Analyst Hardik Parikh highlighted the upcoming phase 1 data readout at Obesity Week in November as a key catalyst, expecting strong efficacy and minimal side effects, with potential to capture ~10% of the U.S. oral obesity drug market. J.P. Morgan also placed Viking under a “positive catalyst watch,” projecting that oral GLP-1 drugs, including VK2735, will play a key role in the $120 billion obesity market by 2030. Viking has strong institutional backing and a promising pipeline, including VK2809 for NASH and VK0214 for X-linked adrenoleukodystrophy.
BIOTECH NEWS
🔹 On Sept. 13, Bavarian Nordic’s mpox vaccine, IMVANEX (MVA-BN; also known as JYNNEOS), has been prequalified by the WHO, marking it as the first mpox vaccine to be added to the WHO prequalification list. This approval, based on data reviewed by the EMA, will facilitate access to the vaccine in African countries heavily affected by the mpox outbreak. The vaccine is approved for use in adults for smallpox and mpox. WHO also recommends its “off-label” use in infants, children, adolescents, pregnant, and immunocompromised individuals during outbreaks.
🔹 On Sept. 12, Noom, a digital health company, announced a new weight loss program offering access to a compounded version of semaglutide, the active ingredient in Wegovy and Ozempic. The program starts at $149, providing a more affordable alternative to branded GLP-1s, which can cost around $1,000 per month. Noom’s compounded option is meant to address the high demand for weight loss drugs and offers flexibility for users to taper off the medication if desired.
🔹 After announcing that its investigational lung cancer drug, ivonescimab, outperformed Keytruda in a phase 3 clinical trial, Summit Therapeutics announced on Sept. 12 it has raised $235M through a private placement of approximately 10.35 million shares at $22.70 per share. The funding was sourced from both insiders and leading biopharma institutional investors. The company plans to use the proceeds to advance the clinical development of ivonescimab, particularly for non-small cell lung cancer and potentially other cancers.
🔹 On Sept. 12, Neurocrine Biosciences announced that its ERUDITE phase 2 trial for luvadaxistat (NBI-1065844) failed to meet its primary endpoint for treating cognitive impairment in schizophrenia. This news follows earlier positive results from the INTERACT study. Due to these outcomes, Neurocrine will discontinue further development of luvadaxistat and shift focus to advancing NBI-1117568 for schizophrenia and NBI-1065845 for major depressive disorder into phase 3 clinical development.
🔹 On Sept. 12, Moderna shared R&D progress and strategy priorities, mentioning that phase 3 trial for skin cancer (melanoma) treatment, mRNA-4157, has almost completed enrollment. While initial discussions with regulators, including the FDA, did not support accelerated approval based on phase 2 data, Moderna and Merck remain committed to advancing the phase 3 trial while continuing to engage with regulatory bodies.
🔹 On Sept. 11, Ascendis Pharma’s YORVIPATH (palopegteriparatide) received Orphan Drug exclusivity from the U.S. FDA, granting it seven years of market exclusivity in the U.S. for treating adult hypoparathyroidism. YORVIPATH, a once-daily prodrug of parathyroid hormone, is designed to offer continuous hormone exposure.
🔹 On Sept. 11, Roche announced early-phase results for its obesity drug CT-966 at a medical conference. According to Reuters, the company revealed that the encouraging outcomes from an initial trial of CT-966 were derived from only 6 patients, highlighting the ongoing uncertainty surrounding the development project. This news follows a 4.5% stock drop two days earlier, triggered by reports of side effects from its other obesity drug, CT-388. Despite the negative press, Hans Clevers, Roche’s head of pharma research, emphasized that CT-388 performs comparably to other drugs in its class and that no patients discontinued treatment due to side effects.
🔹 On Sept. 11, Radiant Biotherapeutics announced it has secured $35M in Series A funding, co-led by the Bill & Melinda Gates Foundation and Amplitude Ventures. The financing will advance Radiant’s Multabody platform, an antibody technology designed to develop multi-functional biologics targeting diseases like cancer, inflammation, and infectious diseases, including HIV. The funds will specifically support the development of Radiant’s lead candidate, 4-1BB, moving it towards clinical trials.
🔹 On Sept. 10, a new study published in NJEM investigated the safety and efficacy of Novo Nordisk’s GLP-1 agonist, Saxenda (liraglutide), in children aged 6 to 12 years with obesity. In this phase 3 trial, 82 children were randomized to receive either daily subcutaneous liraglutide (56 participants) or a placebo (26 participants) alongside lifestyle interventions over 56 weeks. Results showed that children treated with liraglutide had a significantly greater reduction in BMI (-5.8% vs. +1.6%) and body weight compared to the placebo group. A BMI reduction of at least 5% was achieved in 46% of the liraglutide group versus 9% of the placebo group. Adverse events, particularly gastrointestinal, were more common with liraglutide. Overall, liraglutide plus lifestyle interventions effectively reduced BMI in children with obesity.
CLINICAL TRIAL UPDATES
🔹 On Sept. 12, Gilead Sciences announced that its twice-yearly injectable HIV drug, lenacapavir, demonstrated a 96% reduction in HIV infections in a phase 3 trial, PURPOSE 2. The drug proved superior to daily Truvada (a standard oral PrEP) and showed that 99.9% of participants in the lenacapavir group did not acquire HIV. The trial included diverse participants from multiple countries. Based on these promising results, Gilead has ended the blinded phase of the trial and will provide lenacapavir to all participants. Gilead plans to file for regulatory approvals by the end of 2024, with a potential launch in 2025. The company aims to make lenacapavir accessible in high-incidence, low-resource regions and is working on licensing agreements to ensure affordability.
🔹 On Sept. 12, GSK announced positive results from a phase 2 trial of its mRNA seasonal flu vaccine, CureVac, showing strong immune responses against influenza A and B strains in both younger and older adults. The vaccine demonstrated an acceptable safety profile and will move into phase 3. The study involved 500 participants and compared different mRNA vaccine doses to a licensed comparator.
🔹 On Sept. 11, it was announced that Sanofi and Regeneron’s phase 3 trial, Liberty-Cupid Study C, confirmed the effectiveness of Dupixent (dupilumab) in treating chronic spontaneous urticaria, a skin condition causing hives and intense itching. The study involved patients who were unresponsive to standard antihistamines. After six months of treatment, Dupixent reduced itch severity by 8.64 points compared to a 6.10-point reduction in the placebo group. Similarly, Dupixent reduced hive severity by 15.86 points versus 11.21 with placebo. These results support a potential FDA resubmission about a year after the FDA rejected their application.
🔹 On Sept. 11, GSK announced that its early-stage therapeutic herpes simplex virus (HSV) vaccine candidate, GSK3943104, did not meet the primary efficacy objective in the phase 2 portion of the TH HSV REC-003 trial. As a result, the vaccine will not move to phase 3 trials. However, no safety concerns were identified, and the study will continue for routine safety monitoring and follow-up data collection.
🔹 Novo Nordisk’s experimental obesity pill, amycretin, demonstrated promising results in a phase 1 trial, showing a weight loss of up to 13.1% over 12 weeks. The pill, which targets gut hormone GLP-1 and pancreas hormone amylin, exhibited mild-to-moderate gastrointestinal side effects similar to those seen with other incretin-based therapies. While one serious non-fatal event occurred, most side effects were manageable, prompting further development. A decision on whether to proceed directly to a phase 3 trial will depend on upcoming data from a subcutaneous version study. Novo’s shares have surged with its obesity treatments, though they’ve declined recently.
🔹 On Sept. 10, BridgeBio Pharma announced results from the phase 1/2 ADventure trial of BBP-631, an investigational gene therapy for congenital adrenal hyperplasia (CAH). The trial demonstrated that BBP-631 increased endogenous cortisol production in all patients at higher doses, achieving unprecedented results in CAH patients. Additionally, the gene therapy was well tolerated, with no serious adverse events reported. However, the results did not meet expectations for additional capital investment, leading to a significant reduction in the gene therapy budget and the company won’t move forward with the development.
SCIENCE SPOTLIGHT
🔹 Morocco reported its first confirmed case of mpox in the city of Marrakech yesterday (Sept. 12), marking the country’s first detection since the WHO declared the outbreak a global health emergency. The patient, a man, is receiving treatment and is in stable condition. Authorities are tracing his contacts, but no symptoms have been found in others so far. This mpox case comes amid a larger outbreak in Africa, particularly driven by a new variant, though Morocco has not confirmed if this case is linked to the clade Ib variant currently spreading in central Africa.
Read more on the recent mpox outbreak:
🔹 On Sept. 9, JAMA published a groundbreaking study detailing the world’s first successful combined whole-eye and face transplant performed in May 2023 at NYU Langone Health. The patient, Aaron James, a 47-year-old from Arkansas who had lost his eye and much of his face in an electrical accident, underwent this pioneering surgery which lasted 21 hours. The complex procedure involved transplanting not just the eye but also the surrounding facial structures, using advanced microsurgical techniques.
Despite the success in maintaining blood flow and normal pressure in the transplanted eye, and the retina’s responsiveness to light, James has not regained vision, as the optic nerve did not reconnect to the brain. However, the surgery represents a significant step forward in facial and ocular reconstruction.
ON THE HORIZON
🔹 Today marks the first day of the European Society for Medical Oncology (ESMO) Congress, running from Sept. 13-17 in Barcelona, Spain, where many companies are expected to present clinical trial data. For a comprehensive summary, see last week’s ON THE HORIZON:
🔹 Avadel Pharmaceuticals’ LUMRYZ for idiopathic hypersomnia is still awaiting an FDA decision on a supplemental new drug application for pediatric narcolepsy. While the FDA was expected to announce a decision by Sept. 7, Avadel’s narcolepsy drug LUMRYZ remains under review with no decision yet.
Happy Friday and thanks for reading Biotech Blueprint!
DISCLAIMER: This content is for informational purposes only. It should not be taken as legal, tax, investment, financial, or other advice. The views expressed here are my own and do not reflect the opinions of any company or institution.
DISCLOSURE: I have no business relationships with any company mentioned in this article.