This Week in Biotech #09
Catch up on the latest biotech breakthroughs and upcoming trends (Sept 6-10).
Welcome to This Week in Biotech by Biotech Blueprint!
To keep things more digestible, I am introducing shorter biotech updates every Wednesday. More comprehensive Friday posts will continue to feature the Science Spotlight and On the horizon sections, along with the latest breakthroughs, clinical trial results, and pharma news.
MARKET UPDATES
🔹 On Sept. 10, Ionis Pharmaceuticals (IONS) priced its public offering of $11.5M shares at $43.50 per share, raising approximately $500M before expenses. Despite the offering, Ionis’ stock price dropped over 12% yesterday. The company granted underwriters a 30-day option to purchase up to 1.725 million additional shares. The proceeds will be used to fund commercial launches, clinical programs, research, and general corporate purposes. The offering is expected to close on Sept. 11, 2024.
🔹 On Sept. 10, Altimmune’s (ALT) stock rose 9% after the company presented promising mid-stage results for its obesity drug, pemvidutide, at a European diabetes conference. Pemvidutide, a dual GLP-1/glucagon receptor agonist, showed strong results in preserving lean mass and reducing visceral adipose tissue, which is linked to cardiovascular risk.
🔹 On Sept. 10, Corvus Pharmaceuticals’ (CRVS) stock surged nearly 14% after the company announced the initiation of a phase 3 clinical trial for its lead drug, soquelitinib, targeting relapsed or refractory peripheral T-cell lymphoma (PTCL). Soquelitinib, an ITK inhibitor, has received Orphan Drug and Fast Track Designations from the FDA. The trial aims to evaluate its effectiveness in extending progression-free survival compared to existing treatments. Secondary goals include overall survival and response rates.
🔹 On Sept. 10, Centessa Pharmaceuticals (CNTA) saw a 10% rise followed by a decline in its stock after announcing positive interim phase 1 results for its narcolepsy drug candidate, ORX750. The data showed significant improvements in wakefulness and a favorable safety profile. The company plans to advance ORX750 into phase 2 trials for narcolepsy type 1, narcolepsy type 2, and idiopathic hypersomnia in Q4 2024.
🔹 On Sept. 10, Viridian Therapeutics’ (VRDN) shares jumped >30% following positive topline results from the phase 3 THRIVE trial of its lead candidate, veligrotug, for active thyroid eye disease. The trial met both primary and secondary endpoints. Viridian plans to submit a biologics license application to the FDA in the second half of 2025.
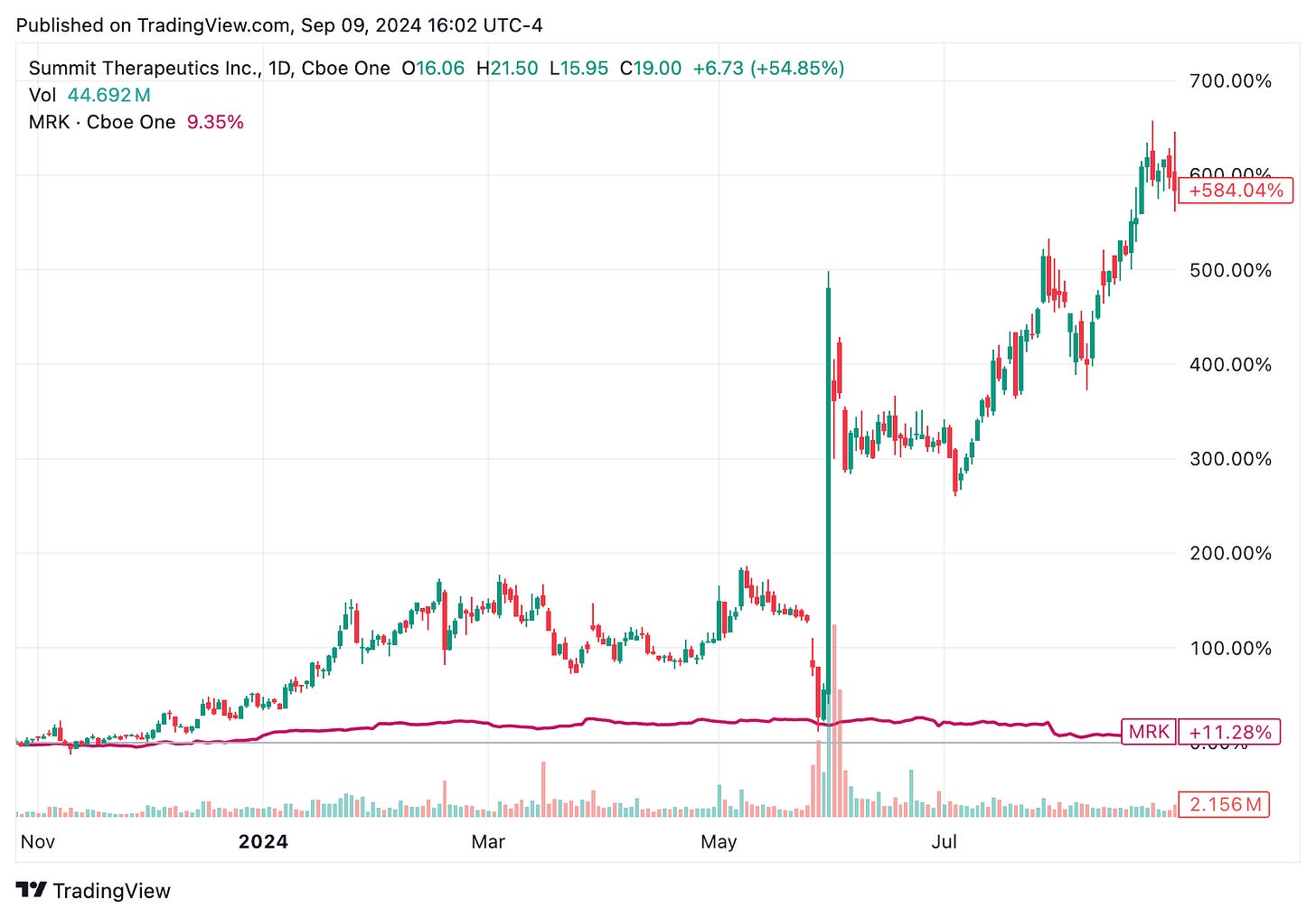
🔹 On Sept. 9, Summit Therapeutics (SMMT) shares surged 56% after its investigational lung cancer drug, ivonescimab, outperformed Merck’s Keytruda in a phase 3 clinical trial. The results of the HARMONi-2 trial were presented on Sept. 8 at the 2024 World Conference on Lung Cancer, showing a median progression-free survival of 11.14 months for ivonescimab, compared to 5.82 months for Keytruda. In response, Merck (MRK) shares dropped 5%. Summit’s stock has surged 625% year-to-date, driven by the initial announcement of the HARMONi-2 trial results in late May 2024.
🔹 On Sept. 9, Tonix Pharmaceuticals’ (TNXP) shares surged 14% following positive results for its potential mpox vaccine, TNX-801. The vaccine demonstrated efficacy in protecting animals from lethal mpox challenge and showed improved tolerability, even in immunocompromised subjects. The vaccine, based on an attenuated horsepox virus, is praised for its single-dose administration and better safety profile compared to older vaccines. These results were presented at Immunology Symposium at the University of Alberta.
🔹 On Sept. 9, Immunovant’s (IMVT) stock dropped 7% following the release of positive data from a phase 2a study of batoclimab for Graves’ disease. The study showed a 76% response rate in patients uncontrolled by antithyroid drugs. On Monday, Citi raised its price target for Immunovant shares to a market-high of $60.00, up from the previous $51.00, while maintaining its Buy rating on the stock.
🔹 On Sept. 9, Relay Therapeutics’ (RLAY) stock surged over 57% following promising interim results for its drug candidate RLY-2608, aimed at treating certain types of advanced or metastatic breast cancer. The study showed that RLY-2608 combined with fulvestrant provided significant progression-free survival and demonstrated a favorable safety profile. Relay plans to begin a pivotal study in 2025.
🔹 On Sept. 9, Rezolute (RZLT) shares jumped 13% after the FDA lifted a partial clinical hold on RZ358 (ersodetug), its therapy for hypoglycemia from congenital hyperinsulinism. The hold was due to liver toxicity observed in rats, but the FDA found it irrelevant to humans. Rezolute will now start U.S. trials for the phase 3 sunRIZE study, aiming for early 2025 enrollment and topline data in the latter half of 2025. The study will include 56 participants across over a dozen countries.
🔹 On Sept. 9, Xencor’s (XNCR) stock jumped 24% following the announcement of new drug development programs and updates on existing oncology candidates. The company revealed four new XmAb drug programs targeting autoimmune diseases, including XmAb942 for inflammatory bowel disease, XmAb657 for autoimmune conditions, and two bispecific T-cell engagers: plamotamab and XmAb657. In oncology, Xencor provided updates on XmAb819, showing initial anti-tumor activity in advanced clear cell renal cell carcinoma, and XmAb808, which is in phase 1 trials for advanced solid tumors. Both are expected to reach target doses by the end of 2024, with clinical updates planned for 2025.
🔹 Evaxion Biotech (EVAX) shares fell 4.5% on Sept. 9 despite reporting positive mid-stage trial results for its cancer vaccine, EVX-01. The phase 2 trial showed a 69% overall response rate, with 11 of 16 patients exhibiting clinical responses and 15 showing tumor reductions. The complete one-year data will be presented at the European Society for Medical Oncology (ESMO) Congress 2024. EVX-01, developed using Evaxion’s AI-Immunology platform, is being tested in combination with Merck’s Keytruda for advanced melanoma.
🔹 On Sept. 6, Travere Therapeutics (TVTX) saw its stock rise in premarket trading after the FDA granted full approval for its kidney disease drug, Filspari. The stock surged by over 10%, building on momentum from the drug’s earlier accelerated approval in February 2023. Full approval, supported by confirmatory results from the PROTECT study, allows broader prescription of the drug, boosting investor confidence in the company’s future growth potential.
BIOTECH NEWS
🔹 On Sept. 10, Gilead Sciences and Genesis Therapeutics announced a strategic collaboration to discover and develop new small molecule therapies using Genesis’s AI platform, GEMS (Genesis Exploration of Molecular Space). Genesis will provide its AI expertise to help generate and optimize molecules against targets selected by Gilead. Gilead will have exclusive rights to develop and commercialize the resulting products. The deal includes an upfront payment of $35M to Genesis for three targets, with additional fees for extra targets. Genesis can also earn milestone payments and royalties on future sales.
🔹 On Sept. 10, Bayer announced the phase 3 OASIS 3 study results support the efficacy and long-term safety of its investigational drug, elinzanetant, for treating moderate to severe hot flashes associated with menopause. The 52-week study showed significant reductions hot flashes frequency and improvements in sleep quality and menopause-related quality of life. The safety profile remained consistent with prior studies, with no signs of liver toxicity or endometrial issues. Elinzanetant is a non-hormonal, once-daily oral treatment. Bayer has submitted a New Drug Application to the U.S. FDA for approval.
🔹 On Sept. 10, Carisma Therapeutics and Moderna announced they have expanded their collaboration to develop two in vivo CAR-M (chimeric antigen receptor macrophage) therapies for treating autoimmune diseases. Building on previous success in oncology, the partnership will utilize Carisma’s CAR-M technology and Moderna’s mRNA/LNP platform. Carisma will oversee discovery, while Moderna will handle clinical development and commercialization. Carisma is eligible for milestone payments and royalties from net sales of any resulting therapies.
🔹 The U.S. House of Representatives passed the Biosecure Act on Sept. 9, 2024, with a decisive vote of 306 to 81, aimed at restricting business with several Chinese biotech firms, including WuXi AppTec, WuXi Biologics, BGI Group, MGI and Complete Genomics. The legislation, which cites national security concerns, prohibits federal contracts with these companies and their affiliates, as well as those that engage in business with them. Proponents argue that the bill is essential for protecting Americans’ health data and ensuring the integrity of the U.S. pharmaceutical supply chain. In response, WuXi AppTec and BGI have denied any security threats, asserting that the legislation is based on unfounded allegations. Following the bill’s passage, shares of WuXi AppTec fell sharply in Hong Kong trading, reflecting investor concerns about the potential impact on their operations in the U.S. The bill now awaits approval from the Senate before being sent to President Biden for final enactment.
🔹 On Sept. 9, GSK presented late-breaking data at the European Respiratory Society Conference showing that their ultra-long-acting biologic, depemokimab, significantly reduces severe asthma exacerbations. In the phase 3 SWIFT-1 and SWIFT-2 trials, depemokimab reduced asthma exacerbations by 54% compared to placebo over 52 weeks. It also led to a 72% reduction in hospitalization or emergency visits. Depemokimab, administered every 6 months, targets interleukin-5 and could be the first ultra-long-acting biologic for asthma. The results were published on Sept. 9 in the New England Journal of Medicine. Safety profiles showed similar rates of adverse events between depemokimab and placebo, with no serious treatment-related issues.
🔹 On Sept. 8, Summit Therapeutics revealed that ivonescimab, a bispecific antibody, significantly reduced the risk of disease progression or death by 49% compared to Keytruda (pembrolizumab), the first-line treatment of PD-L1-positive advanced non-small cell lung cancer (NSCLC). Presented at the 2024 World Conference on Lung Cancer, the phase 3 HARMONi-2 trial demonstrated that patients receiving ivonescimab achieved median progression-free survival of 11.14 months vs 5.82 months for Keytruda. The benefits spanned across various subgroups, including both PD-L1 high and low expressers. Safety profiles were comparable for the two drugs. The results mark ivonescimab as the first drug to outperform Keytruda in a phase 3 trial for NSCLC. Summit plans to initiate another phase 3 trial, HARMONi-7, in 2025. Additional data on ivonescimab will be presented at ESMO 2024 conference coming up on September 13-17 in Spain.
🔹 On Sept. 6, GSK announced its phase 3 MATINEE trial of Nucala (mepolizumab) in chronic obstructive pulmonary disease (COPD) patients showed a significant reduction in moderate/severe exacerbations over 2 years compared to placebo, meeting its primary endpoint. The safety profile was consistent with previous findings. COPD affects over 300 million people globally, with 40% showing type 2 inflammation, driven by elevated eosinophil levels. Nucala, targeting IL-5, may offer a new treatment option for these patients, pending further regulatory review. Full results will be shared at a future scientific congress.
CLINICAL TRIAL UPDATES
🔹 On Sept. 10, Altimmune’s announced that pemvidutide, a dual GLP-1/glucagon receptor agonist, showed strong results in preserving lean mass and reducing visceral adipose tissue, which is linked to cardiovascular risk. In a phase 2 sub-study of 67 subjects, those treated with pemvidutide for 48 weeks experienced a 21.9% reduction in lean mass ratio and a 28.3% decrease in visceral adipose tissue. Pemvidutide’s lean mass preservation is considered superior to traditional diet and exercise methods. The drug is also being studied for metabolic dysfunction-associated steatohepatitis and has received Fast Track designation from the FDA.
🔹 On Sept. 10, Viridian Therapeutics announced positive topline results from the phase 3 THRIVE clinical trial of VRDN-001 (veligrotug), an anti-IGF-1R antibody for treating active thyroid eye disease (TED). The trial achieved all primary and secondary endpoints, demonstrating a 70% proptosis responder rate and significant improvements in other key measures. Veligrotug was well-tolerated with no serious adverse events reported. The ongoing THRIVE-2 trial for chronic TED is expected to provide topline data by year-end 2024, with a biologics license application submission planned for the second half of 2025.
🔹 On Sept. 9, Terns Pharmaceuticals announced positive phase 1 trial results for TERN-601, a once-daily oral GLP-1 receptor agonist for obesity treatment. The trial showed statistically significant mean weight loss of up to 5.5% over 28 days, with a 4.9% placebo-adjusted weight loss at the highest dose (740 mg). TERN-601 was well-tolerated with no treatment-related dose interruptions, reductions, or discontinuations, even with rapid dose titration. The company plans to move into phase 2 trials in 2025.
🔹 On Sept. 9, it was announced that AstraZeneca and Daiichi Sankyo’s datopotamab deruxtecan showed a median overall survival of 14.6 months in the TROPION-Lung01 phase 3 trial in patients with advanced nonsquamous non-small cell lung cancer (NSCLC), compared to 12.3 months with docetaxel. Although the overall survival benefit was significant in the nonsquamous subgroup, it was not statistically significant for the overall trial population. The trial also demonstrated a meaningful progression-free survival advantage with datopotamab deruxtecan, consistent with earlier reports.
🔹 On Sept. 8, J&J presented positive long-term results for its chemotherapy-free combination of Rybrevant-Lazcluze in non-small cell lung cancer patients with certain EGFR mutations. The phase 3 MARIPOSA trial showed that 61% of patients on this regimen were alive after a median follow-up of 31.1 months, compared to 53% for AstraZeneca’s Tagrisso. The combination also showed a trend toward better central nervous system disease control. This comes after the US FDA recently approved the combination as a first-line treatment for NSCLC.
🔹 On Sept. 7, it was announced that an interim analysis of the IDeate-Lung01 phase 2 trial by Merck and Daiichi Sankyo showed promising results for ifinatamab deruxtecan (I-DXd) in treating pre-treated extensive-stage small cell lung cancer (ES-SCLC). The 12 mg/kg dose achieved a 54.8% objective response rate and was selected for further studies. Patients saw a median progression-free survival of 5.5 months and an overall survival of 11.8 months. The safety profile was consistent with previous findings.
Happy Wednesday and thanks for reading Biotech Blueprint!
DISCLAIMER: This content is for informational purposes only. It should not be taken as legal, tax, investment, financial, or other advice. The views expressed here are my own and do not reflect the opinions of any company or institution.
DISCLOSURE: I have no business relationships with any company mentioned in this article.




