Navigating Clinical Trials
What does it mean when a drug enters Phase 1 or Phase 2b clinical trial? How long do these phases take and what is evaluated in each step? What's the likelihood the a drug makes it to the market?
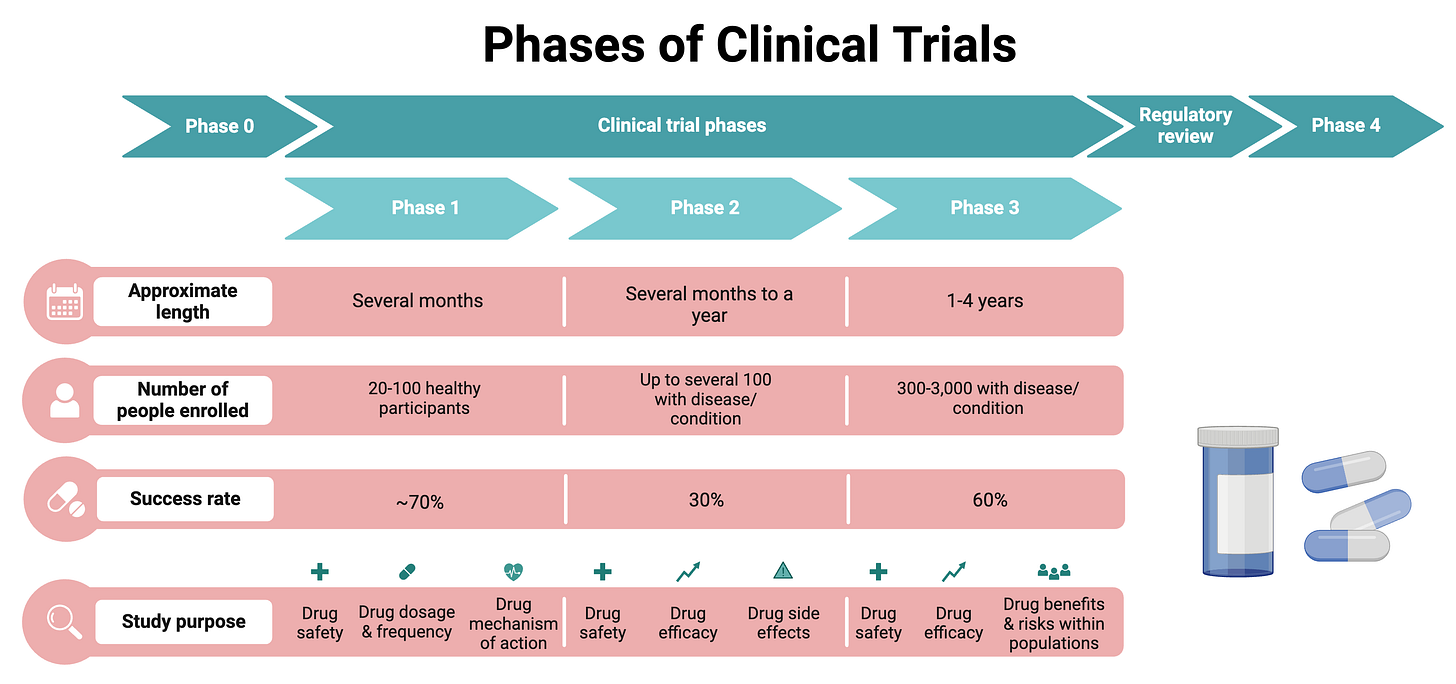
Clinical trials are a critical component of the drug development process, providing the data needed to assess the safety and efficacy of new treatments. The process is highly structured and consists of several stages, each with specific goals and requirements. In my posts, I often refer to Phases or stages of clinical trials, but what does each Phase encompass? Here is a high-level overview of the process:
💊 Preclinical studies
Before human trials can begin, preclinical studies are conducted in the lab and on animal models to evaluate initial efficacy and safety. Potential toxicities and adverse effects of the drugs are also noted. The decision to proceed from preclinical studies to Phase 1 clinical trials is typically made by the pharmaceutical or biotech company developing the drug and this decision is subject to regulatory approval. The company submits an Investigational new drug application to the FDA that includes results from preclinical studies and other information like drug’s composition and targets, and plans for proposed clinical trials.
💊 Phase 0
This phase may be optional and include a few people that are administered the drug in very small doses to gather preliminary data on pharmacodynamics (what the drug does to the body) and pharmacokinetics (what the body does to the drug).
💊 Phase 1: Determining safety and dosage
Phase 1 trials are the first stage of testing in humans. These studies involve a small group of healthy volunteers (20-100) and are primarily focused on assessing the safety, tolerability, pharmacokinetics, and pharmacodynamics of a drug. Volunteers are often compensated for their time as this step usually has little benefit to them. Phase 1 generally takes several months to complete and about 70% of drugs move to the next stage.
In the clinical trial process, phases are sometimes subdivided (e.g., Phase 1a, Phase 1b, Phase 2a, Phase 2b, etc.) to provide more detailed information about the objectives and methods of the trials. For example Phase 1a may include healthy individuals with the main goal to determine a safe dosage range, identify side effects, and understand how the drug is metabolized and excreted. Subsequently, Phase 1b involves patients with the target disease rather than healthy volunteers with a goal to evaluate safety and tolerability in the target patient population and gather preliminary data on efficacy in this group.
💊 Phase 2: Efficacy and side effects
Phase 2 trials involve a larger group of participants (100-300) who have the disease or condition that the drug is intended to treat. These studies aim to obtain preliminary data on the drug’s efficacy - the main question asked is, does the treatment work in treating/preventing a disease X? Only about 20-30% of drugs succeed and move onto Phase 3. Phase 2 can take several months to 2 years.
Again, Phase 2 might divided into 2a and 2b, and while there are no formal definitions of these divisions, 2a can be referred to as “proof-of-concept” and 2b provides more concrete evidence of the drug’s efficacy and safety. (Efficacy and safety are the most important measurements though, assessed at every step of the way during the clinical trial process.)
💊 Phase 3: Confirmation and monitoring
Phase 3 trials involve an even larger group of patients (300-3,000) and aim to confirm the drug’s effectiveness, monitor side effects, compare it to commonly used treatments, and collect information that will allow the drug to be used safely. This stage can take up to 4 years and approximately 60% of drugs that enter Phase 3 get approved. Phase 3 is less likely to be subdivided into subphases.
💊 Regulatory review and approval
After successful completion of Phase 3 trials, the data is submitted to regulatory agencies such as the FDA in a New drug application. This can take several months to years, depending on the drug and the regulatory body. It includes a thorough review of all preclinical and clinical data, manufacturing processes, labeling, and proposed use.
✨ Voilà! The drug is marketed.
💊 Phase 4: Post-market surveillance
“Phase 4” occurs after a drug has been approved for consumer sale. These studies gather additional information on the drug’s risks, benefits, and optimal use. They are designed to monitor the long-term effects and safety of the drug, detect any rare or long-term adverse effects, and assess the drug’s performance in various populations. Phase 4 is technically ongoing as long as the drug is in use.
Why do most clinical trials fail?
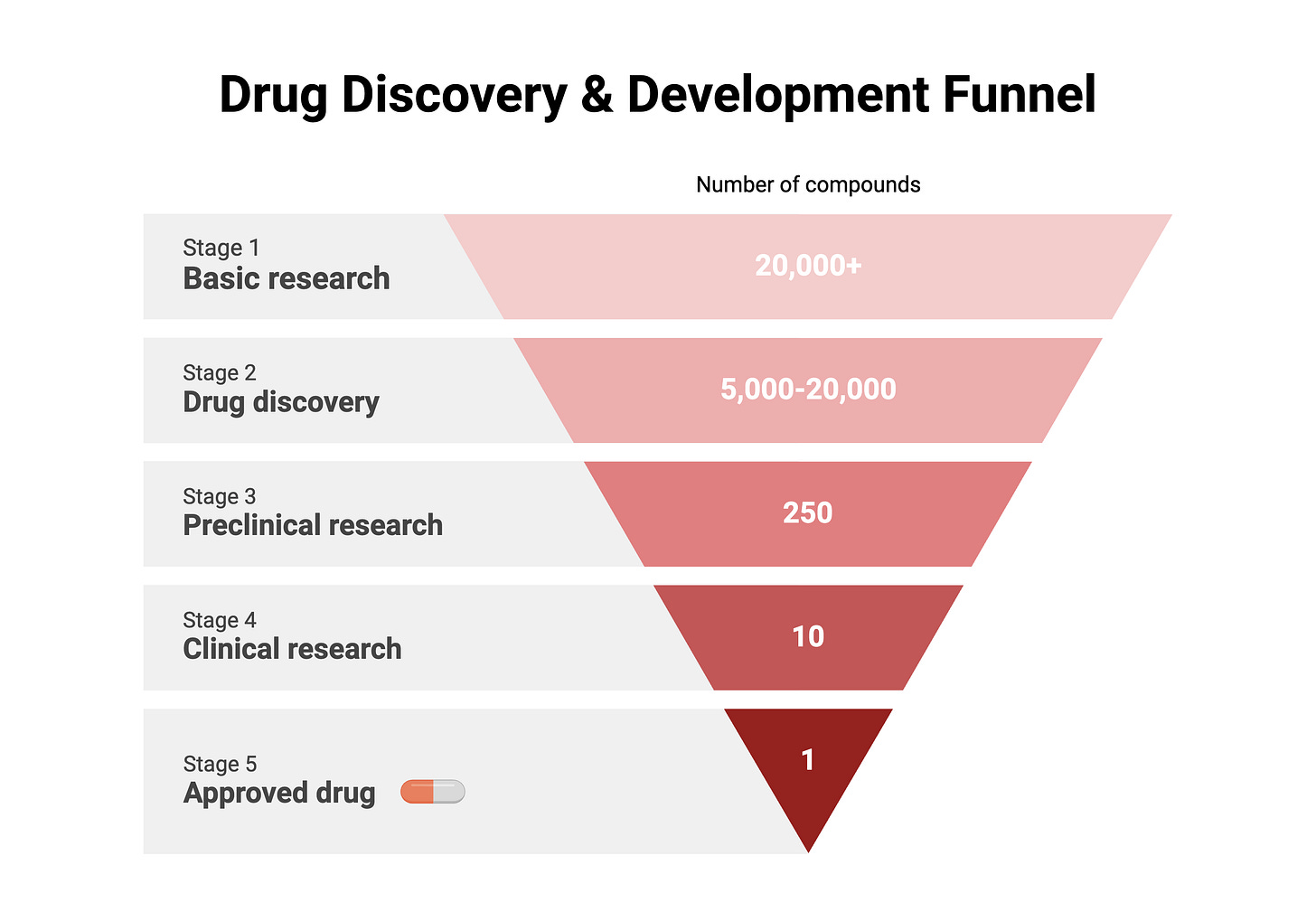
Only a tiny fraction of compounds investigated by basic research scientists make it to clinical trials and about 90% of clinical trials fail, but why? I think there are two broad reasons for this.
1) Drug approval standards have become more stringent over time, resulting in an increase in the average time and money required to test potential new drugs and obtain the necessary data for new drug approval submissions. Also, the cost of R&D has skyrocketed due to the need for more advanced technologies, specialized expertise, and extensive testing to meet regulatory standards. Regulatory guidelines and requirements also change often which can lead to additional hurdles for pharma companies.
2) In the early days of drug development, there were many therapeutic opportunities, particularly in the treatment of infectious diseases and cardiovascular conditions. However, as therapies for these diseases have become widely available today, the easily addressable, or “low-hanging fruit” diseases, are largely gone. The “remaining” diseases are more complex such as neurodegenerative disorders, cancers with high mutation rates, and rare genetic disorders, which are harder to target and treat effectively. Additionally, understanding the underlying biology of diseases is a significant scientific challenge, requiring advanced research methods and technologies that add to the time and cost of drug development.
Why are many drugs that make it all the way to Phase 4 expensive?
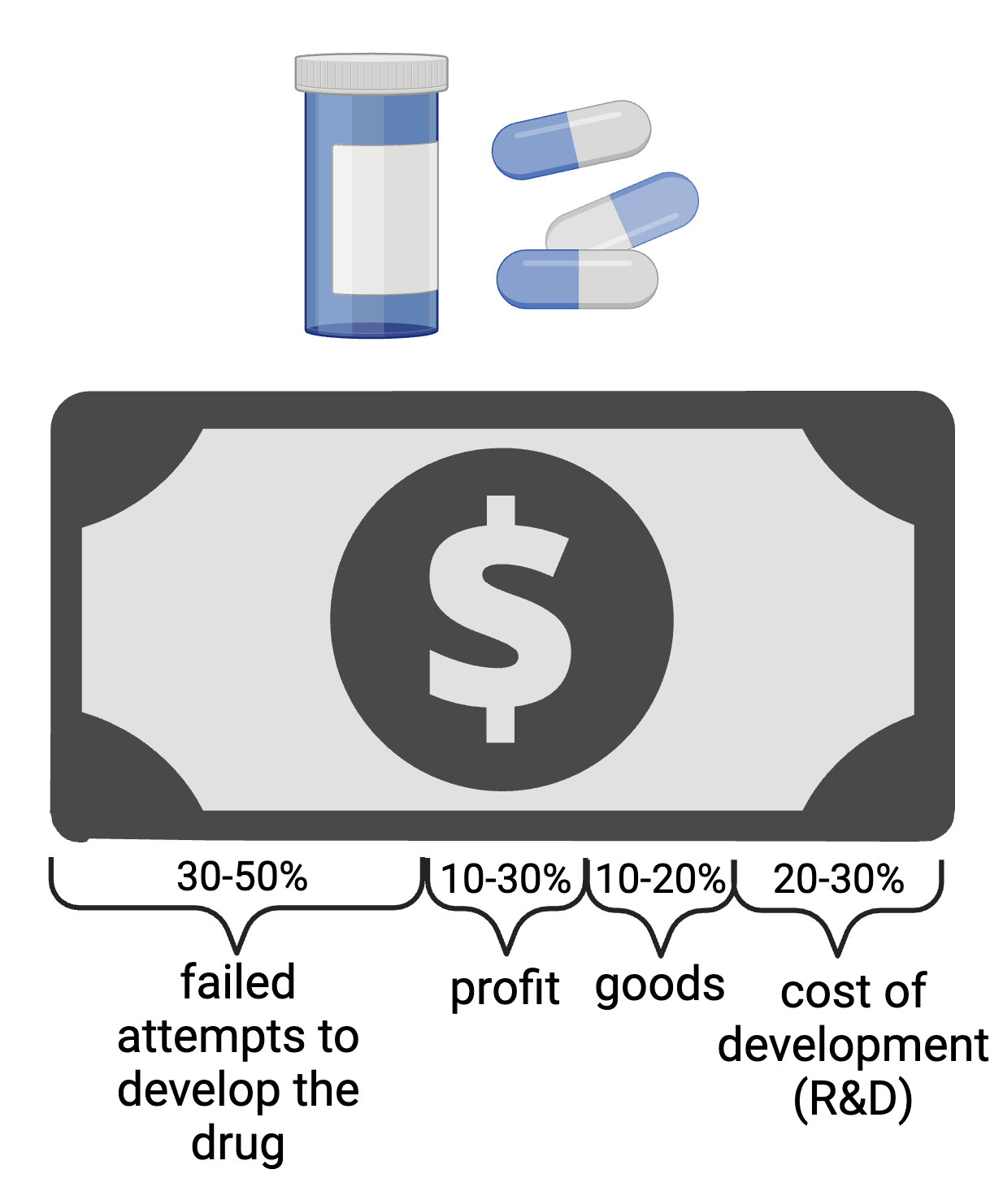
The oversimplified answer is that drugs take a long time to develop and so many efforts fail along the way that need to be reflected in the final price tag. Up to half of the drug cost is associated with failed candidate development. The average cost to develop a new drug, including the cost of failed candidates, is estimated to be around $2.6 billion.
Of course, it’s quite complicated to determine the exact breakdown of the cost of a new drug, but we can divide the major expenses into four categories: failed attempts to develop the drug (30-50% of the final cost), profit (10-30%), cost of goods (10-20%), and costs associated with the actual research and development (20-30%).
This simplified division highlights the primary components contributing to the overall cost of a new drug. The percentages can vary depending on the specific circumstances of the drug and the pharmaceutical company.
How are drug names selected?
Have you ever wondered how are drug names selected? The process is not as simple as it might sound. The pharmaceutical company’s marketing team, often with the help of branding experts, creates several potential brand names. They aim for the names to be distinctive to avoid medical errors, easily pronounceable (though this seems not always achieved), and meaningful, often reflecting the drug’s function or chemical structure. These names also have to be screened for potential legal, cultural, and linguistic issues. Final names are submitted for regulatory approval to agencies like the FDA, the U.S. Adopted Names (USAN) Council and sometimes the WHO to review these names further for safety, clarity, and appropriateness. Once approved, the pharmaceutical company registers the brand name as a trademark to protect it legally. All of this is reflected in the cost of goods of the drug.
New advances in clinical trials and future outlook
Clinical trials are evolving, especially with the advent of technology and artificial intelligence. For example, the integration of digital health technologies, such as wearable devices and mobile health applications, is transforming clinical trials. These technologies enable real-time data collection, remote monitoring, and improved patient engagement, which can enhance the accuracy and efficiency of clinical trials. Also, AI and machine learning are increasingly being used to optimize clinical trial design, patient recruitment, and data analysis. These technologies can identify patterns and predict outcomes more effectively, leading to more efficient and cost-effective trials.
Graphs (Phases of Clinical Trials, Drug Discovery & Development Funnel, and Cost of Drug Breakdown) were created using BioRender.com.